2. Real-time monitoring of 2-amino-2-methylpropan-1-ol and piperazine emissions to air from TCM post combustion CO2 capture plant during treatment of RFCC flue gas (2022)
Audun Drageseta*, Øyvind Ullestada,b, Eirik Romslo Kleppea,b, Blair McMastera,c, Magnus Aronssona,b, Jonas-Andre Olsena,b
aTechnology Centre Mongstad (TCM), 5954 Mongstad, NorwabEquinor ASA, PO Box 8500, 4035 Stavanger, Norway
cTotalEnergies EP Norge AS, Finnestadveien 44, Dusavik, 4029 Stavanger, Norway
Monitoring and understanding the emissions of pollutants is vital for safe implementation of new industries. To ensure the safe adoption of amine-based post-combustion carbon capture to combat climate change, reliable and accurate monitoring technologies must be available for commercial projects to ensure they can monitor and control any new pollutants that might results from capturing CO2 from an industrial flue gas. As a test site for carbon capture technologies, Technology Centre Mongstad (TCM) monitors pollutants emitted in the flue gas via online sampling and analysis as per regulatory requirements. This work presents the first results from a newly installed ion-molecule reaction mass spectrometer (IMR-MS) that was employed during a test campaign with the amine solvent blend of 2-amino-2-methylpropan-1-ol (AMP) and piperazine (PZ) to monitor trace pollutants in the emitted flue gas. The primary pollutants were monitored and reported in real time in the range from 100 ppb (parts per billion) to 10 ppm (parts per million) and compared with extractive isokinetic sampling during a test campaign with an oil refinery cracker gas. The instrument allowed for real-time trending of the amine pollutants AMP and PZ in ppb range, which is the expected range required by regulators for some full-scale plants.
Decarbonizing heavy industries is key for achieving the carbon mitigation goals outlined in the IPCC-6 report [1]. Amine-based carbon capture is among the most mature technologies for decarbonizing existing industrial point sources for CO2 emissions. Technology Centre Mongstad (TCM) have operated and demonstrated both non-proprietary and proprietary amine solvents for post-combustion carbon capture (PCCC) since 2012 [2]. TCM is located on the west coast of Norway in the vicinity of Equinor’s oil refinery at Mongstad. With access to two distinctly different industrial flue gases: combined-cycle gas turbine (CCGT)-based combined-heat-and-power plant (CHP) and RFCC (Residual fluid catalytic cracker) and the ability to manipulate these flue gases through air dilution and recycling of CO2, TCM can assess solvent technologies under conditions that are representative of multiple industries emissions [3]. Among the main objectives of TCMs test campaigns is to reduce the risk (economic and environmental) for commercial application and full-scale deployment of Carbon Capture and Storage (CCS). Key among the test campaigns conducted
* Corresponding author. Tel.: +47 9592 5273, E-mail address: Audun.drageset@tcmda.com
at TCM are the open test campaigns with non-proprietary solvents like aqueous 2-aminoethan-1-ol (commonly known as Monoethanolamine or MEA) and the aqueous blend of 2-amino-2-methylpropan-1-ol (AMP) and Piperazine (PZ), also known as CESAR1. Data and learnings from these campaigns can be disseminated in line with TCMs purpose to ensure safe technology adoption to combat climate change.
The test activities at TCM must be conducted within the framework of an emission permit [4]. The permit regulates aspects of the plant operation, among them emission to air and sets restrictions on components and their concentration. During the CO2 capture process trace amounts of contaminants are introduced to the treated gas emitted to air. Common pollutants are amines and volatile degradation products from the process like aldehydes, ketones and ammonia. Of particular concern is the class of compounds known as Nitramines and Nitrosamines, as these are known mutagenic agents and can cause cancer with prolonged exposure. In addition to direct process emissions, amines generate these compounds through photochemical reactions in the atmosphere [5]. Thus, the combination of stack emission monitoring and dispersion modelling is used to ensure the load to local environment (air and water) are within acceptable safety margins to safeguard public health and avoid damaging local ecosystems. TCM is required by the regulators to conduct continuous real-time monitoring of emissions and similar requirements may be imposed on full- scale plants in Norway and the rest of Europe. Industrial emission monitoring instruments and methods will therefore be important for full scale adoption of amine-based carbon capture. Fourier transformed infrared spectroscopy (FTIR) has been the primary instrument for real-time reporting of emissions for plant operation at TCM, but the quantification limit has been in the range of 0.5-1 ppm [2c]. Instruments with lower limits are required as the emission limit for some plants can be 1 ppm or lower [6]. Proton Transfer Reaction Time of Flight Mass spectrometry (PTR-TOF-MS) have been demonstrated in monitoring applications at TCM down to single digit ppb levels [7], however at time of writing the instrument results are not available in real-time for plant operation as the raw results recorded by the instrument require post-processing by skilled personnel. Herein we present results from a new Ion-Molecule Reaction Mass spectrometer (IMR-MS) tested during the 2020 campaign with an open solvent mixture of AMP and PZ.
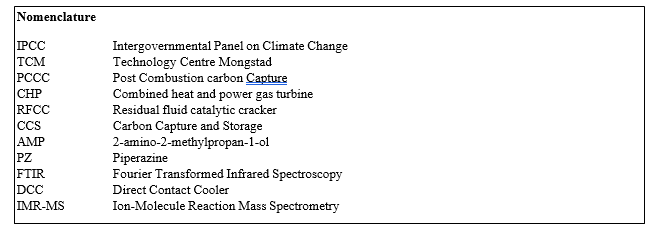
TCM operates a generic amine plant built and designed by Aker Solutions and Kværner with a flue gas capacity up to 60 000 Sm3/h and which has previously been described in the literature, hence only a description of the plant configuration is disclosed below [2]. The data presented in this work is from a test period of optimized plant configuration with the RFCC flue gas (14 vol% CO2) and a solvent mixture of AMP (29 wt%) and PZ (11 wt%). The plant was operated with 18-meter packing height and a flue gas flow rate of 35 000 Sm3. The plant was optimized to reduce aerosol emissions by
(1) removal of acidic sulfuric aerosols from the flue gas via pH regulation of the Direct Contact Cooler (DCC) (NaOH injection to keep pH 8) [8],
(2) Brownian diffusion filter [3b] to further reduce particle count and
(3) a temperature profile adjusted to promote aerosol growth and capture in the first two water washes. The plant configurations and process conditions are summarized in Table and Figure 1, additional information and overall test objectives of the campaign are available in the literature [9].
The RFCC flue gas contain acidic aerosols. Such aerosols will promote amine emissions to air, as amines are dissolved in the aerosol droplets as they travel through the absorber [10]. To acquire representative samples from such an emission point one must account for the aerosol momentum [11]. Key considerations are:
(1) selection of the sample probe shape and placement,
(2) the sampling rate and conditioning of the sample and
(3) its transport.
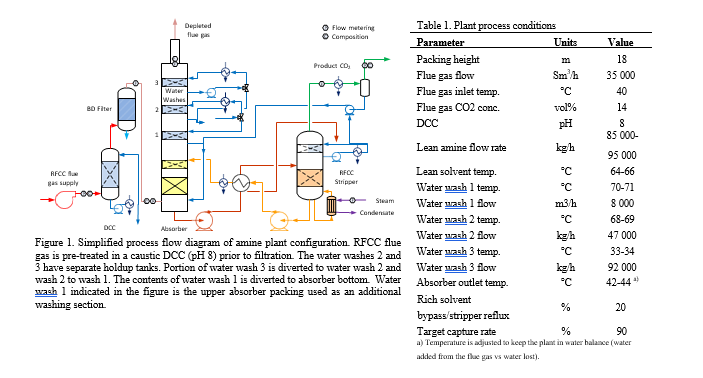
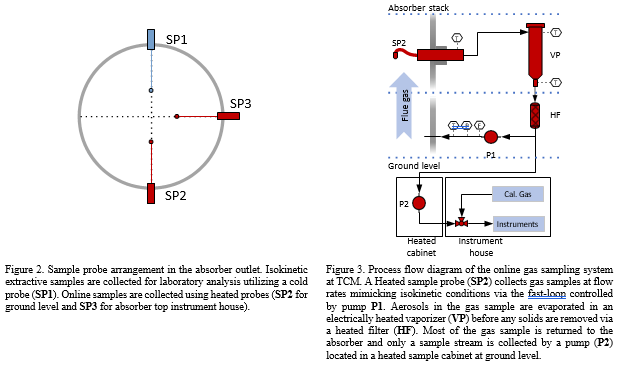
As a test site TCM have additional needs with respect to gas sampling and analysis for R&D applications and have therefore 3 separate sample points with comparable probes and location (Gooseneck, Paul Gothe, Heated combination probe, Version B, S-Pitot tube) (see Figure 2). Isokinetic extractive samples are collected via a cold sample system (see section 2.4) while online systems use a heated sample system (see Figure 3). The heated gas sampling system at TCM is designed to reduce measurement bias when the gas contains aerosols. The sample gas is collected via a gooseneck sample probe (SP2) heated to 120 °C. In addition, a fast-loop system is configured to sample the stack at 1 m3/h, resulting in a sampling velocity 10-11 m/s that mimics isokinetic sampling conditions. By mimicking isokinetic sampling conditions one aim to get a representative sample of the aerosols in the gas. The fast loop is equipped with a vaporizer (Elmess thermo systems, Electrical flow heater, 35 – 200 °C with modified temperature control) where the sample is heated to elevated temperature (120 °C), disrupting and evaporating the aerosols to the gaseous phase. An inline heated filter (3-micron pore size) removes solid particulates from the gas stream. A sample stream of the fast-loop sample gas is transported through an approximately 100-meter sample line (O’Brien, TRACEPAC®, TrueTube EPS, Electropolished SilcoNet tubing, 1/4 inch) to an environmentally controlled analyser-house at ground level for easy access to the analytical instruments. The final heated samples system (SP3) is connected to an analyser
house at the top of the absorber via a 10-meter heated sample line and is predominantly used for research applications [7].
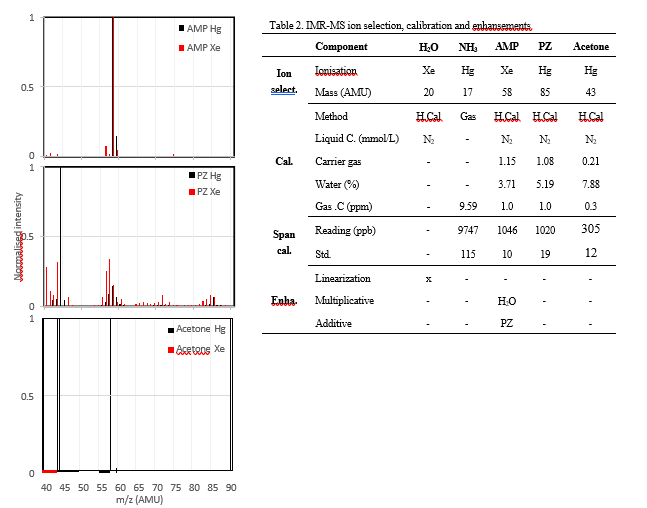
This work utilizes a commercially available Ion-Molecule Reaction Mass spectrometer (IMR-MS) (AirSense, V&F Analyse- und Messetchnik GmbH, Austria) [12]. Soft chemical ionisation of the sample gas is favourable to respectively reduce and minimise fragmentation of the parent molecules in a complex mixture like a flue gas containing trace volatile organic compounds. To achieve this the instrument utilises different gasses with varying ionisation energy to ionise the sample, in this work Hg (10.44 eV) and Xe (12.13 eV) ionisation modes were used. This can allow for the separation of ions with similar mass if their ionisation potential is different. The IMR-MS was installed in the ground level analyser house with the sampling system described in Figure 3. The instrument was calibrated using aqueous solutions and a calibration gas generator (HOVACAL, Digital 312-MF, IAS GmbH, Germany) with nitrogen as a carrier gas (Table 2.). The mass spectra of each component were recorded and used in selection mass signal for monitoring (see Figure 4). AMP exhibited water cross sensitivity in addition to mass fragment overlap with PZ, these effects were minimized after the implementation of enhancements (online calculations to correct for cross interaction between components and non-linear behaviour) in the instrument software suite (V&F Viewer Software, version 2.4). An overview of compound selection and enhancements are found in Table 2.
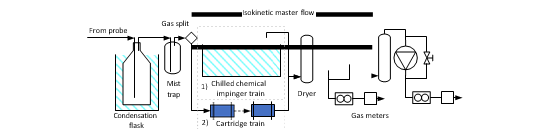
The extractive sampling equipment, control unit (Paul Gothe GmbH) and sample train was assembled at the sample location by TCM laboratory personnel. The probe and sample lines were at ambient temperature with a sample train consisting of three capture modes: (1) Condensation flask which cools down the gas to collect condensable, (2) Mist trap consisting of an empty impinger flask containing a 1 mm jet generating approximate gas speed of 80 m/s that that collects medium/large aerosols and (3) absorption flasks containing sulfuric acid (0.05M) and a 1mm jet captures small aerosols, gaseous amines and ammonia in the acidic solution (see Figure 5). Generally sampling time is 1-2 hours. Samples are analysed using a Dionex Integrion HPIC System (model ICS-5000, Thermo Fisher Scientific) which included an IonPac CS19 column and an IonPac CG19 guard column and MS detector (Thermo Fisher, ISQ EC Mass spectrometer). Aldehydes and acetone are sampled using a similar sample train where the absorption bottles are exchanged with two silica cartridges (Sep-Pak® DNPH-Silica Cartridge, 800 mg). Sample time is considerably shorter as cartridges have lower capacity than absorption flasks, all cartridges were analysed by a third-party lab. Isokinetic gas sampling was performed on five occasions during the test period.
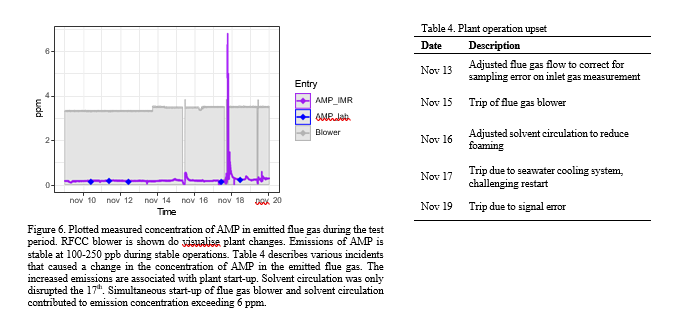
During the test period minor plant upsets occurred resulting in unstable plant conditions in the following hours (see Figure 6 and Table 3). The instruments capture the impact on process emissions prior to reaching a steady state. Shorter process upsets have negligible impact and emissions are relatively stable. However more complex situations or extended downtime can result in a significant elevation of emissions during a limited period. Accommodating for increased emissions during such eventualities should be described in the plant emission permit to ensure regulators and operators are aligned on acceptable limits that give operators the required flexibility to stabilise the plant in such scenarios.
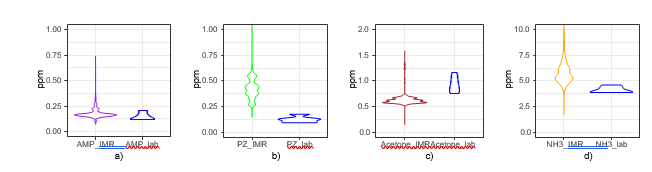
The incidents described above do not represent stable process conditions and are therefore removed from the dataset when comparing the performance of the IMR-MS instrument to established extractive sampling methods. Figure 7 shows violin plots of the cleaned online dataset and extractive lab sampling. Violin plots are similar to box plots but also visualize the distribution of measurements. Among the extractive samples, AMP results have the best agreement with the extractive samples (Figure 7a). As AMP was shown to be the primary emission component in previously
reported work [11], care was taken to implement enhancements to mitigate cross-sensitivity affecting AMP during calibration. The water content in the flue gas was the main component impacting the AMP signal, this was resolved by adding a linearization to the water signal in addition to a multiplicative enhancement with AMP. As seen in Figure 4 PZ has a fragmentation pattern that overlaps with the selected mass for AMP (see Table 2). The impact of this interaction is reduced with an enhancement (Table 2), as a result AMP is monitored with high accuracy.
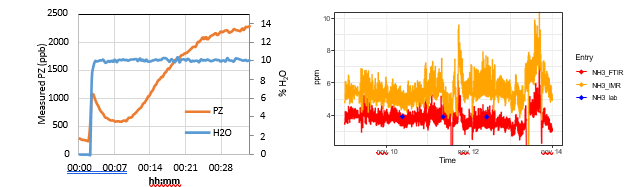
The calibration of PZ was challenging when using the available aqueous solutions and Hovacal setup. The component is sticky and adheres to the surfaces of the calibration equipment and lines. Therefore, achieving saturation of the Hovacal and inlet lines to the instrument were time consuming (30-60 minutes). A potential consequence is that a zero or span calibration was performed before the system had reached equilibrium, as is suggested in Figure 8, pointing to a bias in the span calibration. This could explain the instrument bias shown in Figure 7b.
Figure 7c shows poor agreement between the IMR-MS and the extractive samples for acetone. No clear explanation could be discerned by examining the calibration logs or setup. Further investigation is required.
Ammonia on the IMR-MS overestimates the concentration as can be seen in Figure 7d, however this is due to a calibration bias as is evident when comparing with FTIR results (see Figure 9). The instruments follow the same trends, and the bias should be resolved by improved the instrument calibration.
In this work we have successfully demonstrated the application of a new IMR-MS instrument for real-time monitoring and reporting of contaminants in the treated flue gas for an amine-based post-combustion carbon capture process. The instrument was installed in an industrial environment and measured the main emission components regulated in the emission permit of TCM.
The real-time monitoring and reporting of 2-methyl-2-aminopropan-1-ol (AMP) emissions was successfully conducted with a new IMR-MS instrument at TCM. The instrument accurately reported AMP in a range of 100-250 ppb. The instrument also reported PZ, acetone and ammonia but with lower accuracy. This was predominantly due to sub optimal calibration setups and procedures resulting in a systematic error. To mitigate some of the challenges experienced in this work dry calibration gasses should be used, preferably certified gas bottles. In addition, a more thorough screening of the flue gas contaminants and potential cross interactions of all contaminants should be assessed. This work demonstrates that commercial instruments capable of monitoring emissions in ppb range are available on the market. With application tailored calibration the instrument can be adapted to different flue gas sources and capture solvents.
The authors gratefully acknowledge the staff of TCM DA, Gassnova, Equinor, Shell and TotalEnergies for their contribution and work at the TCM DA facility. The authors also gratefully acknowledge Gassnova, Equinor, Shell, and TotalEnergies as the owners of TCM DA for their financial support and contributions.
IPCC, 2021: Summary for Policymakers. In: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [MassonDelmotte, Zhai VP, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, and Zhou B (eds.)]. Cambridge University Press.
Gjernes E, Pedersen S, Jain D, Åsen KI, Hvidsten OA, de Koeijer G, Faramarzi L, de Cazenove T, Documenting modes of operation with cost saving potential at the Technology Centre Mongstad, 14th Greenhouse Gas Control Technologies Conference Melbourne 21-26 October 2018 (GHGT-14), b) Faramarzi L, Thimsen D , Hume S, Maxon A, Watson G, Pedersen S , Gjernes E, Fostås BF, Lombardo G, Cents T, Morken AK, Shah MI, de Cazenove T, Hamborg ES, Energy Procedia, 114, 2017, 1128-1145, c) Morken A, Pedersen S, Kleppe ER, Wisthaler A, Vernstad K , Ullestad Ø , Flø NE, Faramarzi L, Hamborg ES, Energy Procedia, 114, 2017, 1245-1262, d) Benquet C, Knarvik A, Gjernes E, Hvidsten OA, Kleppe ER, Akhter S, First Process Results and Operational Experience with CESAR1 Solvent at TCM with High Capture Rates (ALIGN-CCUS Project), Proceedings of the 15th Greenhouse Gas Control Technologies Conference 15-18 March 2021.
Johnsen K, Kleppe ER, Faramarzi L, Benquet C, Gjernes E, de Cazenove T , Morken AK, Flø N, Shah MI, Aronsson M, Ullestad Ø, CO2 product quality: assessment of the range and level of impurities in the CO2 product stream from MEA testing at the Technology Centre Mongstad (TCM), Proceedings of the 14th Greenhouse Gas Control Technologies Conference Melbourne 21-26 October 2018 (GHGT-14), b) Lombardo G, Shah MI, Fostås B, Hvidsten OA, Faramarzi L, de Cazenove T, Lepaumier H, Rogiers P, Results from testing of a Brownian diffusion filter for reducing the aerosol concentration in a residual fluidized catalytic cracker flue gas at the Technology Centre Mongstad, 14th Greenhouse Gas Control Technologies Conference Melbourne 21-26 October 2018 (GHGT-14).
TCM emission permit, Permit Nr.: 2011.0257T, Plant Nr.: 1263.0105.01 – accessed 02.08.2022. https://www.norskeutslipp.no/WebHandlers/PDFDocumentHandler.ashxdocumentID=577603&documentType=T&companyID=25735&aar=0&epslanguage=no
Nielsen CJ, Herrmann H, Weller C, Chem. Soc. Rev., 41, 2012, 6684-6704, b) Låg M, Andreassen Å, Instanes C, Lindeman B, Health effects of amines and derivatives associated with CO2 capture, Norwegian Institute of Public Health, 2011, c) Gjernes E, Helgensen LI, Maree Y, Energy Procedia, 37, 2013, 735-742, d) de Koeijer G, Talstad VR, Nepstad S, Tønnesen D, Falk-Pedersen O, Maree Y, Nielsen CJ, 2013, 18, 200-207, e) Tan W, Zhu L, Mikoviny T, Nielsen CJ, Wisthaler A, D’Anna B, Antonsen S, Stenstrøm Y, Farren NJ, Hamilton JF, Boustead GA, Brennan AD, Ingham T, Heard DE. J. Phys. Chem. A, 125, 2021, 411-422, f) Tan W, Zhu L, Mikoviny T, Nielsen CJ, Tang Y, Wisthaler A, Eichler P, Müller M, D’Anna B, Farren NJ, Hamilton JF, Petterson JBC, Halliquist M, Antonsen S, Stenstrøm Y, J. Phys. Chem. A, 125, 2021, 7502-7519.
Tønnesen D, CO2-capture at Klemetsrud. Modelling of nitros- and nitramines, Norwegian Institute for Air Research, NILU report 11/2018. https://hss.miljodirektoratet.no/api/1/publisert/hoering/vedlegg/16832, accessed 20.07.20
Languille B, Drageset A, Mikoviny T, Zardin E, Benquet C, Ullestad Ø, Aronson M, Kleppe ER, Wisthaler A, Best practices for the measurement of 2-amino-2-methyl-1-propanol, piperazine and their degradation products in amine plant emissions, 15th Greenhouse Gas Control Technologies Conference Abu Dhabi 15-18 March 2021 (GHGT-15), b) Languille B, Drageset A, Mikoviny T, Zardin E, Benquet C, Ullestad Ø, Aronsson M, Kleppe ER, Wisthaler A, Atmospheric emissions of amino-methyl-propanol, piperazine and their degradation products during the 2019-20 ALIGN-CCUS campaign at the Technology Centre Mongstad, 15th Greenhouse Gas Control Technologies Conference Abu Dhabi 15-18 March 2021 (GHGT-15).
Akinpelumi K, Saha C, Rochelle GT, Piperazine aerosol mitigation for post-combustion carbon capture, International Journal of Greenhouse Gas Control, 2019, 91, 102845.
Hume, SA McMaster, Drageset A, Shah, MI, Kleppe ER, Results from CESAR1 testing at the CO2 Technology Centre Mongstad. Verification of Residual Fluid Catalytic Cracker (RFCC) baseline results, 16th Greenhouse Gas Control Technologies Conference Lausanne 23-27 October 2022 (GHGT-16).
a) de Cazenove T, Bouma RHB, Goetheer ELV, van Os PJ, Hamborg ES, Aerosol Measurement Technique: Demonstration at CO2 Technology Centre Mongstad, Energy Procedia, 2016, 86, 160-170, b) Lombardo G, Fostås BF, Shah MI, Morken AK, Hvidsten OA, Mertens J, Hamborg ES, Results from aerosol measurement in amine plant treating gas turbine and Residue Fluidized Catalytic Cracker flue gases at the CO2 Technology Centre Mongstad, 13th Greenhouse Gas Control Technologies Conference Lausanne 14-18 November 2016 (GHGT-13).
Lodge JrJP, Methods of Air sampling and Analysis, Third edition, CRC Press, 1989.
Sauer C, Lorén A, Schefer A, Carlsson P-A, On-Line Composition Analysis of Complex Hydrocarbon Streams by Time-Resolved Fourier Transform Infrared Spectroscopy and Ion−Molecule Reaction Mass Spectrometry, Analytical Chemistry 2021, 93, 13187-13195, b) Wang Y, Han H, Shen S, Li J, Wang H, Chu Y, Control of solvent use in medical devices by proton transfer reaction mass spectrometry and ion molecule reaction mass spectrometry, Journal of Pharmaceutical and Biomedical Analysis, 2009, 50, 252-256, c) Dearth MA, Evaluation of a Commercial Mass Spectrometer for Its Potential To Measure Auto Exhaust Constituents in Real Time, Industrial & Engineering Chemistry Research, 1999, 38, 2203-2209.
