Results from CESAR1 testing at the CO2 Technology Centre Mongstad. Verification of Residual Fluid Catalytic Cracker (RFCC) baseline results (2022)
Scott A. Humeb*, Blair McMastera,c, Audun Drageseta, Muhammad I. Shaha and Eirik R.
Kleppea,d, Matthew Campbella
aTechnology Centre Mongstad, 5954 Mongstad, Norway
bElectric Power Research Institute, Inc., 1300 West WT Harris Blvd, Charlotte, NC 28262, USA
cTotalEnergies EP Norge AS, Finnestadveien 44, Dusavik, 4029 Stavanger, Norway
d Equinor ASA, PO Box 8500, 4035 Stavanger, Norway
Abstract
Technology Centre Mongstad (TCM DA) was established in 2012 to test, verify, and demonstrate different post-combustion capture (PCC) of carbon dioxide technologies. The company is a joint venture between Gassnova (on behalf of the Norwegian state), Equinor, Shell, and TotalEnergies with a common vision for testing and research and development of carbon capture for the deployment of large-scale carbon capture. The facility is located next to the Equinor refinery in Mongstad, Norway and is provided flue gas from a nearby combined cycle gas turbine-based heat-and-power (CHP) plant as well as residue fluid catalytic cracker (RFCC) flue gas from the refinery.
The CESAR1 solvent, developed in the EU CESAR project, was utilized for the first time at TCM for the ALIGN CCUS (Accelerating Low Carbon Industrial Growth Through Carbon Capture Utilization and Storage) project test campaign in 2019. CESAR1 solvent is a blend of 27% wt 2-amino-2-methylpropan-1-ol (AMP) and 13% wt piperazine (PZ). This solvent is considered a better solvent than monoethanolamine (MEA) in terms of thermal energy performance and stability (lower degradation) and has been proposed by the IEAGHG as their new benchmark solvent. TCM DA carried out baseline testing of CESAR1 solvent in June 2020 using flue gas from the CHP source, controlled at 5% CO2 to simulate state of the art gas turbine flue gas, and continued with additional testing that lasted into late 2020 using flue gas from the RFCC source that has higher CO2 concentration. The main objectives of these campaigns were to produce knowledge and information that can be used to reduce the cost as well as technical, environmental, and financial risks of commercial-scale deployment of PCC using the CESAR1 solvent. This includes the establishment of an RFCC baseline performance with CESAR1 solvent.
The Electric Power Research Institute, Inc. assessed the performance of the CESAR1 process using an independent verification protocol (IVP) previously developed for TCM DA during CHP baseline testing MEA solvent. The IVP was also previously updated for use with the RFCC flue gas as this gas contains 13–14% CO2 content whereas the CHP flue gas has ~3.5% CO2 content by volume. The IVP provides a structured testing procedure for assessing the thermal and environmental performance of PCC processes under normal operating conditions. During the RFCC testing, TCM DA manually collected extractive samples from the depleted gas outlet and the product CO2 outlet throughout the testing period. As part of the IVP, EPRI also assessed plant critical instrumentation at TCM DA for accuracy and precision error based on a comparative analysis during testing operations and against calibration checks.
The CESAR1 baseline process was evaluated during twelve individual test periods over three days in November 2020. During the tests, extractive samples were taken to measure process contaminants such as aldehydes, ketones, amines (AMP/PZ), and ammonia. The extractive sampling and associated analysis techniques applied were consistent with the IVP recommendations. Sulfur oxides and nitrogen oxides were continuously monitored using Fourier transform infrared (FTIR) analyzers on the depleted flue gas and the product CO2 streams. Multiple measurements of the CO2 concentration (FTIR, non-destructive infra-red, and gas chromatography) are available at TCM allowing comparative confirmation of test period stability. The capture rate was calculated via four methods. CO2 recovery (mass balance) was evaluated, and the thermal performance (energy consumption) was assessed based on measured data taken during the tests.
The CO2 capture rate achieved during the testing was about 91%, providing specific reboiler duties (SRD) of 3.2–3.3 GJ/t-CO2 and the CO2 gas mass balance closures were close to 100%. These data and assessments, along with the results from TCM DA sampling during these tests, will be presented in this paper and provide a baseline case for CESAR1 solvent in higher concentration flue gas capture cases.
1. Introduction
The CO2 Technology Centre Mongstad (TCM) is located next to the Equinor refinery in Mongstad, Norway. TCM DA is a joint venture owned by Gassnova representing the Norwegian state, Equinor, Shell, and TotalEnergies. TCM is home to one of the largest post-combustion CO2 capture (PCC) test centres in the world and recently marked the completion of 10 years of technology testing. This facility entered the operational phase in August 2012 and is dubbed the “amine plant.” A unique aspect of the facility is that either a flue gas slipstream from a natural gas-fired combined- cycle gas turbine (CCGT) based combined heat-and-power (CHP) plant or an equivalent volumetric flow from a residual fluid catalytic cracker (RFCC) unit can be used for CO2 capture. The CHP flue gas contains about 3.5% CO2 and the RFCC flue gas contains about 13–14% CO2, the latter of which is comparable to CO2 levels seen in a coal- fired flue gas.
The amine plant, designed and constructed by Aker Solutions and Kværner, is a highly flexible and well- instrumented facility that can accommodate a variety of technologies with capabilities of treating flue gas streams of up to 60,000 Sm3/hr. The plant is offered to developers of solvent-based CO2 capture technologies to test the performance of their solvent technology and to verify technologies aimed to reduce the atmospheric emissions and environmental impact of solvent emissions and degradation products from these processes.
The objective of TCM DA is to test, verify, and demonstrate CO2 capture technologies suitable for deployment at full scale. A significant number of vendors have already successfully tested using the TCM DA facilities to assess their CO2 capture technologies. Multiple tests using the CESAR1 solvent have been carried out at TCM to define the baseline performance of the solvent for defined operating conditions using RFCC flue gas at 13.5% CO2 content in accordance with an independent verification protocol (IVP), which provides a structured testing procedure, developed by the Electric Power Research Institute, Inc. (EPRI) [1].
2. Amine Plant
The TCM 234 tonnes-CO2/day amine plant was designed to be flexible to allow testing of different process configurations. The amine plant is configured to remove CO2 from a natural gas-fired combustion turbine-based CHP plant flue gas or a RFCC off-gas. The typical characteristics of the RFCC gas stream is shown in Table 1.
Table 1. Nominal characteristics of flue gas supplied to TCM
| Parameter | Units | RFCC Flue Gas |
| Temperature | °C | 27 |
| N2+Ar | % vol, dry | 82.5 |
| O2 | % vol, dry | 4.3 |
| CO2 | % vol, dry | 13.2 |
| SO2 | ppmv, dry | 20 to 60 |
| NO | ppmv, dry | 50 to 115 |
| NO2 | ppmv, dry | 3 |
| SO3 | ppmv, dry | 7 to 10* |
| CO | ppmv, dry | 0 to 3 |
| NH3 | ppmv, dry | 1 |
| Particulates | mg/Nm3 | 14 to 41* |
| Chloride | mg/Nm3 | < 0.1 |
* controlled via Brownian demister filter
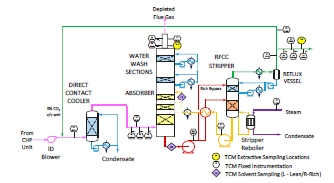
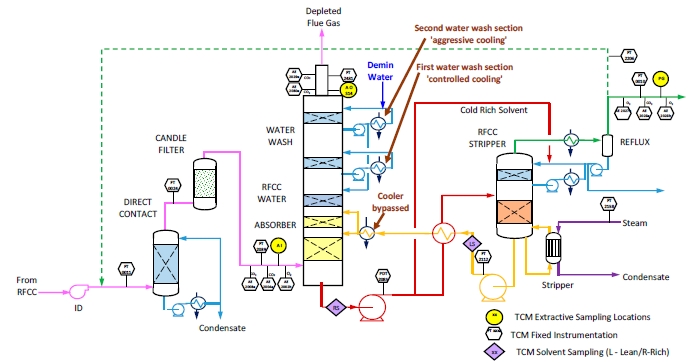
The RFCC type flue gas was used for the tests performed in this work. A process flow diagram showing high-level equipment contained within the amine plant along with key existing instrumentation is shown in Figure 1.
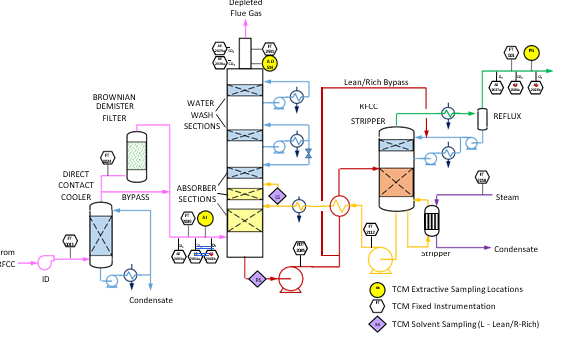
Major system components include:
- An induced draft (ID) blower to overcome pressure drops and blow the flue gas through the plant with an output capacity of up to 270 mbar and 60,000 Sm3/hr.
- A direct-contact cooler (DCC) system to initially lower the temperature of and saturate the incoming flue gas
by a counter-current water flow to improve the efficiency of the absorption process and provide pre-scrubbing of the flue gas. The DCC system has two individually operated packed columns for operations with the CHP flue gas and the RFCC flue gas, respectively. When operating with RFCC flue gas, including the tests reported in this paper, caustic (NaOH) is injected under pH control with a target pH of 8 to reduce levels of acidic SO2 aerosols.
- A Brownian demister module designed to remove particulate and aerosol particles from the flue gas when
using the RFCC. The module has a bypass line to facilitate controlled aerosol levels entering the downstream absorber unit.
- An absorber to remove CO2 from the flue gas using multiple packed sections. The absorber also has water- wash systems located in the upper region of the tower to scrub and clean the flue gas, particularly of any solvent
carry over. When operating with RFCC flue gas, the upper water wash is operated with maximum cooling duty, the lower water wash with minimum cooling duty and additionally, the upper 6 m of absorber packing is utilized as a 3rd “once through” water wash section. The CO2 depleted flue gas passes through a mesh mist eliminator before exiting the absorber column to the atmosphere through a stack located at the top of the column.
- Stripper columns to recover the captured CO2 and return CO2-lean solvent to the absorber. The amine plant consists of two independent stripper columns with a common overhead condenser system. The two stripper
columns are operated independently considering the CO2 content in the flue gas due to column design, hydraulics, and gas velocity effects. Both strippers circulate solvent to the reboilers by thermosiphon. The RFCC stripper also has a circulating pump to assist at low-load operation and during startup. The RFCC reboiler is a shell-and-tube arrangement.
- Balance of plant units include a lean-solvent trim cooler, pumps used to move the CO2-lean and CO2-rich solvent streams between the absorber and stripper, a cross heat exchanger to recover heat from the lean stream, a
reflux drum, stripper condenser, and pumps to dry the product CO2 that exits the stripper.
More details of these components are available in Hume et al [2].
The TCM facility can test virtually any PCC solvent-based process as the amine plant has been designed to accommodate a variety of technologies. The facility also has excellent instrumentation and an on-site lab for detailed analysis.
An IVP was developed to be used as part of the overall performance assessment of amine-based processes and has been updated over time to apply to either CHP or RFCC operation on the TCM amine facility. The IVP is designed to provide a structured testing procedure for assessing thermal and environmental performance of PCC processes under normal operating conditions.
3. CESAR1 RFCC Campaign Overview
The CESAR1 solvent, developed in the EU CESAR project [3], was utilized for the first time at TCM for the ALIGN CCUS (Accelerating Low Carbon Industrial Growth Through Carbon Capture Utilization and Storage) project test campaign in 2019 [4,5]. CESAR1 is a blend of 27% wt 2-amino-2-methylpropan-1-ol (AMP) and 13% wt piperazine (PZ). This solvent is considered a better solvent than monoethanolamine (MEA) in terms of thermal energy performance and stability [6,7] and has been proposed by the IEAGHG as their new benchmark solvent [6]. TCM DA carried out baseline testing of CESAR1 solvent in June 2020 using flue gas from the CHP source, controlled at 5% CO2 to simulate state of the art gas turbine flue gas [2]. During the baseline testing of CESAR1 solvent with RFCC flue gas reported in this paper the average measured solvent concentration was 28.8 / 10.4% wt AMP/PZ.
RFCC flue gas capture performance assessment periods were conducted in November 2020. During the testing, personnel from TCM DA manually collected extractive samples from the depleted gas outlet and the product CO2 line downstream of the RFCC stripper. In previous tests, this was sometimes performed by an independent testing
contractor. However, TCM DA’s competency related to performing this testing was deemed adequate by EPRI during prior MEA baseline campaigns, especially since TCM DA is not commercially involved in the outcome and hence can be considered to be unbiased.
While EPRI was unable to travel to the site to observe TCM’s in-house sampling campaign for the CESAR1 solvent firsthand as a result of travel restrictions due to the SARS-CoV-2 pandemic, several meetings were conducted remotely during the planning phase of the test campaign and EPRI received the data for the tests from TCM DA for analysis. Data logs for all sampling periods containing pertinent flows, temperatures, pressures, and concentrations measured by permanent plant instruments were supplied by TCM DA for the entire test period. The sampling time periods, and sampling period designators are shown in Table 2 along with additional sampling undertaken on each day.
Table 2. CESAR1 RFCC Sampling Periods
| Date | Start Time – Stop Time | Stream Sampled | Sampling Results Reported | Test Period |
| 9:16 – 9:36 | Product CO2 | Aldehydes/Ketones | C7-1 | |
| 17 November 2020 | 9:21 – 9:41 | Absorber Outlet | Aldehydes/Ketones | C7-2 |
| 9:43 – 11:47 | Product CO2 | AMP, PZ, NH3 | C7-3 | |
| 9:54 – 11:58 | Absorber Outlet | AMP, PZ, NH3 | C7-4 | |
| 9:17 – 9:37 | Product CO2 | Aldehydes/Ketones | C7-5 | |
| 18 November 2020 | 9:40 – 11:40 | Product CO2 | AMP, PZ, NH3 | C7-6 |
| 9:51 – 10:11 | Absorber Outlet | Aldehydes/Ketones | C7-7 | |
| 10:24 – 12:31 | Absorber Outlet | AMP, PZ, NH3 | C7-8 | |
| 9:09 – 9:29 | Product CO2 | Aldehydes/Ketones | C7-9 | |
| 22 November 2020 | 9:30 – 9:50 | Absorber Outlet | Aldehydes/Ketones | C7-10 |
| 9:32 – 11:39 | Product CO2 | AMP, PZ, NH3 | C7-11 | |
| 11:00 – 12:00 | Absorber Outlet | AMP, PZ, NH3 | C7-12 |
4. Instrument assessment
To determine the process plant performance, a key component is the quality of the instrumentation installed for measuring the respective compositions and flow rates. Instrumentation quality is determined using two parameters:
Accuracy/bias: This represents the difference between the instrument reading (or average of a set of readings under unchanging process conditions) being assessed and the true value of the parameter being measured. Appropriate determination of the “true value” must be achieved by simultaneous measurement of the parameter using a reference method or instrument with calibration that can be traced to primary standards.
Precision: A determination of the variability of the instrument reading when stream conditions are known to be steady state. Precision is therefore a measure of the random error associated with the measurement.
These measurement errors can be combined to assess the aggregate uncertainty in each measurement. In the absence of a calibration against primary standards for the entire measurement range needed, the uncertainty published by the instrument supplier represents only the precision error.
When the process parameter being measured does not change, precision is a measure of repeatability. In real plant situations, when attempting to operate at steady-state conditions, often process parameters (flow, pressure, and temperature) do vary over the measurement period. Thus, measurements over long periods of time (greater than process time constants) will also include an error term related to process uncertainty.
5. Gas Phase Composition
5.1 CO2 Measurement of Flue Gas Supply and Depleted Flue Gas
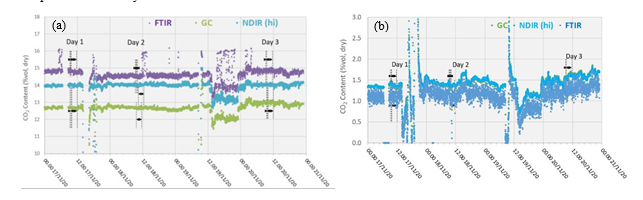
Figure 2 (a) displays the RFCC flue gas supply CO2 concentration data over the test campaign periods. The FTIR data have been corrected to be on a dry basis using the FTIR moisture assessment. The FTIR is biased 1% high and the GC is biased over 1% low in comparison to the HNDIR. Note that the CO2 concentration of RFCC flue gas is beyond the measurement range for the LNDIR and hence cannot be used for these evaluations. As per previous campaigns, the HNDIR data were used in preference to the FTIR and GC data as the NDIR instruments exhibit a higher precision due to a lower measurement spread for the same sampled conditions.
Figure 2 (b) shows the depleted flue gas CO2 measurements with the FTIR instrument included though it showed an excessive precision error. The GC and NDIR instruments agree well regarding changes in gas composition throughout each test day, typically to within 0.01% vol (dry). Although the GC was in good agreement with the NDIR(Hi), it showed a slightly lower precision, therefore as with prior tests, the NDIR(Hi) measurements were used for performance analysis for these tests.
5.2 Oxygen Measurement of Flue Gas Supply and Depleted Flue Gas
Figure 3 shows both the supply and the depleted flue gas oxygen measurements with the GC for the RFCC test period. As the capture rate was constant throughout the period, the concentrating effect, i.e., from 6% vol to 7% vol, for all other components can be clearly seen from the oxygen measurements.
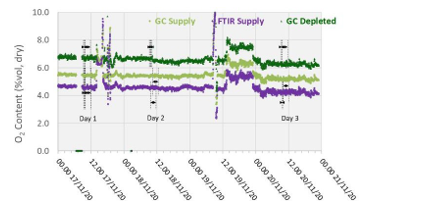
Variations in the inlet oxygen are represented with a similar response on the outlet stream. However, the apparent low precision error exhibited by this commensurate response cannot be determined as each measurement point is from the same core instrument (i.e., variation could indeed be from the instrument and not the process).
6. Gas Phase Flow Rates
6.1 Flue Gas Supply Flows
The RFCC flue gas supply flow is measured by two independent multi-point pitot tube instruments. FIC-0024-ACC and FT-2039, with FIC-0024-ACC attaining a lower precision uncertainty as shown in Table 3. The flow data for the test periods is shown in Figure 4.
Table 3. Key flue gas supply flow instrumentation
| Stream | Tag Number | Instrument Type | Primary Flow Measurement | Precision Uncertainty CHP / RFCC | |
| RFCC Flue Gas Supply | FIC-0024-ACC | Multi-pitot tube | Differential pressure | – | 2.59% |
| Flue Gas Supply at Absorber Inlet | FT-2039 | Multi-pitot tube | Differential pressure | 5.27% | 5.32% |
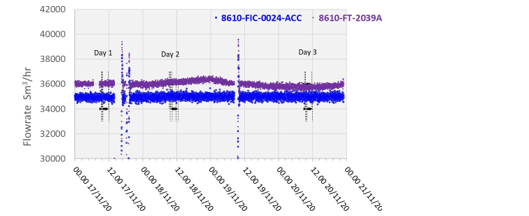
All calculations for CESAR1 RFCC flue gas tests are based on the measured RFCC flue gas flow using FIC-0024.
6.2 Depleted Flue Gas Flows
The depleted flue gas flow was measured by a single multi-pitot tube flow meter whose characteristics are listed in Table 4. Actual measurements depict poor accuracy showing consistent and variable negative bias, as shown in Figure 5, which is likely from plugging in the pitot tube elements caused by the condensation due to the super-saturated moisture condition of the depleted flue gas at this location. This instrument was therefore not utilized to determine the resultant absorber outlet flow. Instead, the conservation of inert components from the inlet stream (oxygen, nitrogen, and argon) was used to determine the appropriate flowrate achieved.
Table 4. Key depleted gas flow instrumentation
| Stream | Tag Number | Instrument Type | Primary Flow Measurement | Precision Uncertainty CHP / RFCC | |
| Absorber Outlet Depleted Flue Gas | FT-2431 | Multi-pitot tube | Differential pressure | 5.28% | 5.38% |
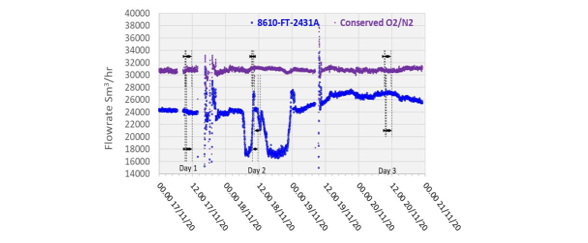
All performance calculations are based on the conserved oxygen and nitrogen method from the flue gas supply measurement as the depleted flue gas flow indication (FT-2431) was shown to not be accurate due to inconsistent measurements with a substantial negative bias due to the saturated flue gas at the absorber outlet.
6.3 Product CO2
The product CO2 flow is normally measured by the vortex flow meter (FT-0010) and the precision uncertainty for this instrument are listed in Table 5.
Table 5. Key product CO2 gas flow instrumentation
| Stream | Tag Number | Instrument Type | Primary Flow Measurement | Precision Uncertainty CHP / RFCC | |
| Product CO2 | FT-0010 | Vortex | Flowing volume | 1.04% | 1.03% |
TCM installed an additional Coriolis flow meter instrument (FT-2215), which previously offered agreement with and exhibited a higher precision than FT-0010 during prior MEA baseline testing. However, this instrument has not undergone an accuracy study and so the precision uncertainty cannot be defined for these tests. The data from both flow meters are shown in Figure 6 for reference. The product CO2 flow was relatively steady throughout the test periods.
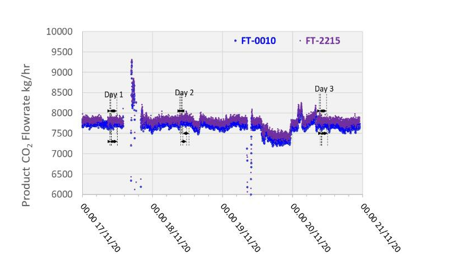
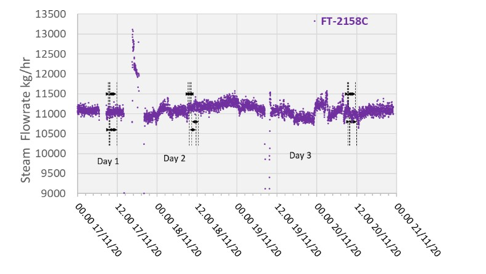
6.4 Steam/Condensate
High-pressure (HP) steam is delivered from the refinery to the TCM amine plant at a pressure of approximately 30 bar, superheated to 240–310°C. The HP steam is throttled to a pressure near the stripper reboiler steam pressure (approximately 4 barg) and then desuperheated with condensate. The stripper reboiler condensate collects in a receiver from which it is returned to the refinery. A small amount of medium-pressure (MP) steam is reduced to a lower pressure for use in steam heat tracing. The low-pressure (LP) steam condensate is returned to the same receiver as the stripper reboiler condensate. The reboiler steam flow rate for the CESAR1 RFCC tests is shown in Figure 7.
7. Results & Discussion
7.1 CO2 Capture Efficiency/Recovery
CO2 capture efficiency can be quantified in several ways depending on how measurements have been taken and the expected accuracy of each individual measurement. EPRI commonly uses the four individual methods that are indicated in Table 6.
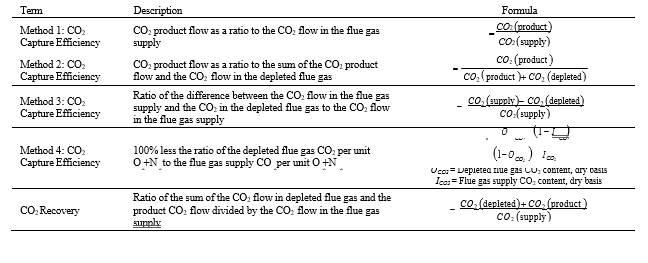
These methods can rely on combinations of the available information to determine a capture efficiency, using the measured gas flowrates in combination with the CO2 analyser measurements. Method 4 simplifies the measurement uncertainty by utilizing only CO2 concentration data and making the well-founded assumption that all incoming inert gases (such as nitrogen and oxygen) will be unchanged through the absorption process. Hence, Method 4 can be used to compare against the other methods that utilize the flow measurements. In addition, the “CO2 Recovery” calculation is indicated in Table 6. The CO2 Recovery is a measure of the CO2 mass balance, being the fraction of CO2 mass flow in the flue gas supply that is accounted for by measured CO2 mass flows in the depleted flue gas and product CO2. It is therefore a measure of the degree to which the CO2 mass balance is closed.
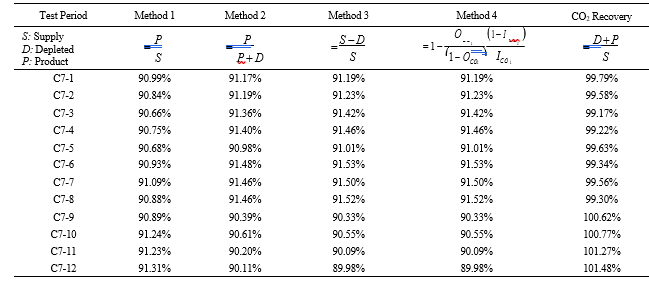
Table 7 shows the four calculation methods of CO2 capture and recovery for the test periods. Note that CO2 product flow can be based on either the measured CO2 product flow (P) or by using the difference between the NDIR-measured CO2 supply and depleted flows (S – D). CO2 capture rates calculated by Methods 2, 3, and 4 were in good agreement within each test period, with Method 1 giving a consistently slightly higher value for CESAR1 CHP testing. It should be noted that Methods 3 and 4 are equivalent due to using the conserved oxygen and nitrogen method for outlet gas flow determination. The CO2 recovery (mass balance) was slightly above 100% for the RFCC tests.
Table 7. CO2 capture results
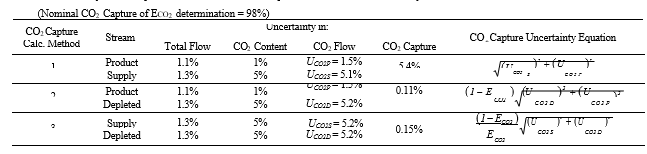
he uncertainty in measurement of flow and composition propagates into the uncertainty in the CO2 capture. The uncertainty calculations and representative results from each of the calculation methods are shown in Table 8. The following assumptions were used:
- Flow metering uncertainties are those theoretically estimated and calculated by internal work at TCM DA for the indicated flow meters
- Concentration uncertainties for the flue gas flows are those described in previously in Section 6
- Concentration uncertainty for the product CO2 is arbitrarily assigned to be 1% vol, which allows for actual CO2 content as low as 99% vol
- CO2 capture of 90% is representative of that measured during all test periods
- The uncertainty in CO2 capture (ECO2) is almost all due to uncertainty in CO2 content of the flue gas supply for the assigned total (low) flow uncertainties. The CO2 capture uncertainty is relatively insensitive to both the product CO2 content uncertainty and the depleted flue gas CO2 content uncertainty.
7.2 Thermal Use
The thermal results are detailed in Table 9. The reboiler heat duty or the heat released in the reboiler is calculated as the difference between steam enthalpy at reboiler inlet and the saturated water enthalpy at the reboiler condensate temperature. The specific thermal use is then calculated by dividing the reboiler heat duty by the product CO2 flow. The two corresponding values for specific thermal energy consumption are shown in results and were consistent during all test periods.
Table 9. Stripper reboiler thermal use calculation
| Test Period | Reboiler Steam Flow Rate kg/hr | Reboiler Heat Duty MJ/hr | Using Product CO2 Flow (P) | Using CO2 Removed from the Flue Gas (S – D) | ||
| Product CO2 Flow kg/hr | Specific Thermal Use GJ/t-CO2 | Captured CO2 kg/hr | Specific Thermal Use GJ/t-CO2 | |||
| C7-1 | 11062 | 25222 | 7790 | 3.24 | 7806 | 3.23 |
| C7-2 | 11059 | 25214 | 7778 | 3.24 | 7811 | 3.23 |
| C7-3 | 11044 | 25177 | 7772 | 3.24 | 7838 | 3.21 |
| C7-4 | 11045 | 25179 | 7776 | 3.24 | 7837 | 3.21 |
| C7-5 | 11098 | 25266 | 7822 | 3.23 | 7850 | 3.22 |
| C7-6 | 11174 | 25413 | 7846 | 3.24 | 7898 | 3.22 |
| C7-7 | 11170 | 25413 | 7845 | 3.24 | 7879 | 3.23 |
| C7-8 | 11179 | 25415 | 7844 | 3.24 | 7899 | 3.22 |
| C7-9 | 11130 | 25246 | 7750 | 3.26 | 7702 | 3.28 |
| C7-10 | 11048 | 25065 | 7790 | 3.22 | 7730 | 3.24 |
| C7-11 | 10992 | 24995 | 7775 | 3.21 | 7678 | 3.26 |
| C7-12 | 10978 | 24977 | 7775 | 3.21 | 7662 | 3.26 |
Previous testing at TCM [8] using conventional 5M MEA solvent (30% wt) with RFCC flue gas at approximately 190 tonnes per day load yielded a regeneration energy range of 3.43–3.55 GJ/t-CO2 using the product CO2 flow (P) and 3.51–3.65 GJ/t-CO2 using the gas-side difference method (S – D), all carried out at a nominal 91% capture rate. The CESAR1 RFCC tests achieved circa 187 tonnes per day load and delivered a regeneration energy range of 3.21-
3.26 GJ/t-CO2 using product flow (P) and a very consistent 3.21-3.28 GJ/t-CO2 using gas-side difference method (S – D). The capture rate was a commensurate 90% for these tests, allowing direct comparison with the MEA tests. The CESAR1 regeneration energy at the same nominal load and capture rate had between 8-10% lower regeneration energy than 5M MEA. The liquid-gas ratio (L/G) for the CESAR1 testing, 2.7 kg/Sm3, was lower than the 5M MEA testing,
3.8 kg/Sm3 due to the higher cyclic capacity of the CESAR1 solvent.
A notable difference between the conditions of the RFCC flue gas testing with 5M MEA and CESAR1 was the flue gas inlet temperature to the absorber. A temperature of 31oC was used for the 5M MEA testing, however due to precipitation in the lower absorber packing, the flue gas temperature was raised to 40oC with CESAR1 to allow stable operation [5]. This increased flue gas temperature for CESAR1 is likely to incur a regeneration energy penalty relative
to testing at the same temperature as 5M MEA. Benquet et al, reported results from early TCM testing with CESAR1 solvent and CHP flue gas including regeneration energy penalty for higher flue gas temperatures due to precipitation issues [5].
7.3 Process Contaminats
Formaldehyde, acetaldehyde, and acetone concentrations were determined by extractive sampling during the CESAR1 RFCC test periods, as shown in Table 10.
Table 10. Depleted flue gas aldehyde/ketone concentrations and mass rates
| Test Period | Formaldehyde | Acetaldehyde | Acetone | ||||||
| ppmvd | mg/Sm3 | g/h | ppmvd | mg/Sm3 | g/h | ppmvd | mg/Sm3 | g/h | |
| C7-2 | 0.286 | 0.36 | 10.39 | 0.099 | 0.18 | 5.27 | 0.779 | 1.91 | 54.72 |
| C7-7 | 0.254 | 0.32 | 9.31 | 0.157 | 0.29 | 8.44 | 1.158 | 2.84 | 82.10 |
| C7-10 | 0.298 | 0.38 | 10.79 | 0.144 | 0.27 | 7.65 | 1.030 | 2.53 | 72.13 |
The formaldehyde levels are substantially higher than the previous MEA RFCC baseline testing measured concentration of 0.06 mg/Sm3. The acetaldehyde levels are considerably lower with CESAR1 than the MEA RFCC test samples of 1.7 mg/Sm3. Acetone levels measured during the MEA tests were sufficiently low at or below the detection limit of 1 mg/Sm3, while with CESAR1 they were between 1–3 mg/Sm3. CO2 product results are shown in Table 11.
Table 11. Product CO2 aldehyde/ketone concentrations and mass rates
| Test Period | Formaldehyde | Acetaldehyde | Acetone | ||||||
| ppmvd | mg/Sm3 | g/h | ppmvd | mg/Sm3 | g/h | ppmvd | mg/Sm3 | g/h | |
| C7-1 | 0.077 | 0.098 | 0.41 | 0.173 | 0.32 | 1.35 | 3.111 | 7.64 | 32.00 |
| C7-5 | 0.075 | 0.095 | 0.40 | 0.153 | 0.29 | 1.20 | 2.701 | 6.64 | 27.89 |
| C7-9 | 0.088 | 0.112 | 0.47 | 0.191 | 0.36 | 1.48 | 3.210 | 7.89 | 32.85 |
For the CO2 product, the formaldehyde levels detected were 2x higher the manual sampled measurements during the MEA RFCC baseline campaign (0.06 mg/Sm3) and the acetaldehyde levels are considerably lower than the previous level of 6.2 mg/Sm3. Unlike the MEA tests, acetone was easily detected in the CO2 product, whereas in the previous MEA baseline all measurements taken were below the detection limit of 0.9 mg/Sm3.
The data suggests that formaldehyde and acetone are generated from the CESAR1 solvent at higher relative rates to acetaldehyde than is the case for MEA.
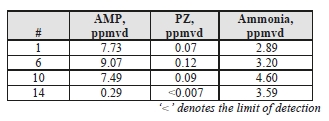
TCM DA measured concentrations of solvent components (AMP and PZ) along with ammonia during the CESAR1 testing, results of these manually extracted samples are shown in Table 12.
Table 12. Depleted flue gas stream ammonia and solvent component concentrations and mass rates
| Test Period | AMP | PZ | Ammonia | ||||||
| ppmvd | mg/Sm3 | g/h | ppmvd | mg/Sm3 | g/h | ppmvd | mg/Sm3 | g/h | |
| C7-4 | 0.113 | 0.426 | 12.23 | 0.090 | 0.33 | 9.41 | 4.590 | 3.31 | 94.91 |
| C7-8 | 0.203 | 0.765 | 22.04 | 0.174 | 0.63 | 18.26 | 4.310 | 3.10 | 89.41 |
| C7-12 | 0.232 | 0.875 | 24.91 | 0.102 | 0.37 | 10.58 | 4.925 | 3.55 | 101.0 |
The solvent components of CESAR1 appear to show substantially lower vapor pressure than is associated with MEA solvent. PZ concentrations were lower than AMP, and the combined AMP/PZ concentrations are ~10 times lower than the MEA equivalent case of 2.9 ppmvd [8]. Ammonia levels are far lower than the MEA tests results, suggesting that ammonia does not represent a significant degradation product of CESAR1.
Extractive solvent and ammonia samples were taken from the CO2 product, and the results are shown in Table 13. In this instance, the AMP measurements were higher than the MEA samples, that were below the detection limit. PZ was detected in 2 of 3 samples, but was present at very low concentrations. Ammonia desorption into the product gas is almost 2 orders of magnitude lower than the depleted flue gas levels, it is comparable with the ammonia concentration detected from the MEA RFCC tests.
Table 13. Product CO2 ammonia and solvent component concentrations and mass rates
| Test Period | AMP | PZ | Ammonia | ||||||
| ppmvd | mg/Sm3 | g/h | ppmvd | mg/Sm3 | g/h | ppmvd | mg/Sm3 | g/h | |
| C7-3 | 0.357 | 0.922 | 5.62 | 0.020 | 0.07 | 0.30 | 0.120 | 0.09 | 0.36 |
| C7-6* | 0.013 | 0.034 | 0.21 | <0.01 | <0.04 | <0.15 | <0.20 | <0.14 | <0.61 |
| C7-11 | 0.523 | 1.351 | 8.24 | 0.005 | 0.02 | 0.08 | 0.119 | 0.09 | 0.36 |
* ‘<’ indicates sample measured below detection limit
Test C7-6 has abnormally low solvent and ammonia levels, not consistent with online measurements. Sulfur dioxide and nitrogen oxide components were measured using FTIR, as shown in Table 14. Prior SO2 measurements for the MEA campaign were under <1 ppmv and this test period was similar. The NO gas appears to largely pass through the absorption process without significant interaction with the solvent. However, the majority of NO2 entering the absorber will react with the solvent as reported by Campbell et al [9].
Table 14. Depleted flue gas SO2 and NO concentrations and mass rates
| Test Period | SO2 | NOx (as NO2) | ||||
| ppmvd | mg/Sm3 | g/h | ppmvd | mg/Sm3 | g/h | |
| C7-1 | 0.04 | 0.11 | 3.1 | 107.7 | 209.6 | 5,994 |
| C7-2 | 0.06 | 0.15 | 4.4 | 108.2 | 210.4 | 6,019 |
| C7-3 | 0.12 | 0.34 | 9.6 | 107.2 | 208.5 | 5,986 |
| C7-4 | 0.14 | 0.38 | 10.9 | 107.1 | 208.5 | 5,985 |
| C7-5 | 0.41 | 1.10 | 31.8 | 108.6 | 211.2 | 6,080 |
| C7-6 | 0.43 | 1.17 | 33.7 | 107.6 | 209.3 | 6,034 |
| C7-7 | 0.21 | 0.58 | 16.8 | 110.6 | 215.2 | 6,213 |
| C7-8 | 0.50 | 1.35 | 39.0 | 107.2 | 208.6 | 6,008 |
| C7-9 | 0.02 | 0.04 | 1.2 | 116.2 | 226.1 | 6,442 |
| C7-10 | – | – | – | 117.4 | 228.4 | 6,511 |
| C7-11 | 0.00 | 0.00 | 0.1 | 117.0 | 227.7 | 6,487 |
| C7-12 | 0.00 | 0.00 | 0.1 | 117.1 | 227.9 | 6,491 |
- Comparison to earlier TCM RFCC CESAR1 Testing
7.4 Comparison to earlier TCM RFCC CESAR1 Testing
The EPRI verification period in November 2020 was the last section of a longer TCM test campaign with CESAR1 solvent and RFCC flue gas. From parametric testing earlier in this campaign, not verified by EPRI, TCM identified a test condition, B4REP2, with an optimum SRD of 3.06 GJ/t-CO2 for 90% capture rate testing with 18m absorber packing height. This case was selected to be repeated during the EPRI verification period. From TCM internal analysis, both overall and CO2 mass balance closure during the B4REP2 test were within 2%.
During the EPRI verification period, higher SRD values than the original B4REP2 test were observed. The major contributing factor to this difference is likely to be foaming in the stripper, which can incur significant SRD penalties as shown in CESAR1 CHP baseline testing. During the EPRI verification period, repeated addition of antifoam had very limited remedial effect. This led to the solvent circulation rate being increased from 85,000 to 95,000 kg/h which reduced but did not eliminate the foaming behaviour. This increase in solvent circulation rate moved the testing during the EPRI verification period away from the optimum L/G ratio identified earlier in the TCM test campaign and incurred an SRD penalty, as described in Table 15 where an additional test condition at 95,000 kg/h from earlier in the campaign, B1, is also included for comparison.
Table 15 – Comparison of CESAR1 RFCC EPRI Baseline Verification Period with earlier TCM CESAR1 RFCC Testing
| EPRI Baseline | B4REP2 Test | B1 | ||
| Date | 17/11/2020 – 20/11/2020 | 02/11/2020 | 24/10/2020 | |
| Lean Solvent Circulation | kg/hr | 95,000 | 84,988 | 94,455 |
| SRD | GJ/t-CO2 | 3.23 | 3.06 | 3.13 |
| Capture Rate | % | 91 | 89.6 | 89.52 |
| Solvent AMP:Piperazine Ratio | Mass Basis | 2.77 | 2.61 | 2.58 |
| Solvent Iron Content | mg/l | 1.36 | 1.02 | 0.98 |
Another contributing factor to the higher-than-expected SRD value is variation in concentration and quality of the CESAR1 solvent due to preferential losses of the piperazine component particularly due to reaction with NO2 as reported by Benquet et al [5], as shown in Table 15. During the EPRI verification period, the average measured solvent concentration was 28.8 / 10.4% wt AMP/PZ compared to the target concentration of 27 / 13% wt AMP/PZ.
7.3 Comparison to pilot and demonstration sites reported in literature
SRD results from previous pilot or demonstration scale testing with CESAR1 solvent with similar capture rate and flue gas CO2 concentration to this TCM EPRI verified baseline are shown in Table 16 with a range of packing materials, solvent concentrations and plant configurations used across the various sites. From the reported results, foaming issues do not appear to have been as prevalent at other sites when compared to TCM.
Table 16 – SRD Results from CESAR1 Pilots at 90% Capture Rate and 13-15% CO2
| Inlet CO2 | Packing | Packing Type | Capture | SRD | Solvent Conc. | Notes | |
| Site | mol%,d | m | – | % | GJ/ t | % wt | – |
| TCM RFCC Baseline | 14% | 18 | Koch Glitsch Flexipac | 91% | 3.23 | 28.8 / 10.4 | |
| RWE Niederaussem [10] | 15% | 16 | Not reported | 90% | 2.97 | 27 / 13 | Intercooler |
| Kaiserslautern [11] | 10% | 4.25 | Sulzer BX 500 | 90% | 3.3 | 28 / 17 | |
| CO2 SEPPL [12] | 13% | 12 | Raschig Superpak 250y | 90% | 3.18 | 28 / 17 | |
| Esbjerg [13] | 13.6% | 16 | Sulzer Mellapak 250X | 90% | 3.1 | 25 / 15 |
The SRD results reported in this paper are within but towards the upper range of the results reported by other CESAR1 test sites. From this comparison, recommendations for future CESAR1 testing at TCM should include the effect of absorber intercooling, mitigation measures for foaming behavior and variation of the piperazine content in the solvent within the precipitation limits.
8. Conclusions
The CESAR1 baseline with RFCC flue gas comparison to the earlier 30% wt MEA baseline performed at TCM DA using the same flue gas source [8] is shown in Table 17.
Table 17. Comparison of TCM DA RFCC baseline conditions and results with CESAR1 and MEA solvents
| MEA Baseline 30% wt | CESAR1 Baseline 28.8 / 10.4% wt AMP/PZ | Notes | ||
| Date | May 2018 | November 2020 | ||
| Flue Gas | RFCC | RFCC | ||
| Packing Height | m | 18 | 18 | |
| Flue Gas Flow | Sm3/h | 35,000 | 35,000 | |
| CO2 Capture Rate | % | 91 | 91 | |
| Flue Gas Temperature | oC | 31 | 40 | Higher due to CESAR1 precipitation limitation |
| SRD | GJ/tCO2 | 3.55 | 3.23 | 9% decrease from CESAR1 to MEA |
| kg/Sm3 | 3.8 | 2.7 | Higher capacity of CESAR1 solvent. CESAR1 L/G not optimized due to foaming. | |
| Liquid:Gas Ratio | ||||
| Stripper Bottom Temperature | oC | 121 | 121 |
The CESAR1 baseline case was conducted at conditions as close to the MEA baseline as possible using the same flue gas source along with an identical packing height, flue gas flowrate, targeted capture rate and the stripper regeneration temperature. The CESAR1 solvent did exhibit precipitation in the absorber packing when the flue gas temperature was too low, hence the higher inlet temperature. The liquid to gas ratio is lower than MEA and was determined based on the solvent characteristics and performance during the tests. The SRD achieved using CESAR1 solvent was 9% lower than what was possible using 30% wt MEA solvent in the same configuration.
Acknowledgements
The authors gratefully acknowledge the staff of TCM DA, Gassnova, Equinor, Shell and Total for their contribution and work at the TCM DA facility. The authors also gratefully acknowledge Gassnova, Equinor, Shell, and TotalEnergies as the owners of TCM DA for their financial support and contributions.
References
- Thimsen D, Maxson A, Smith V, Cents T, Falk-Pedersen O, Gorset O, Hamborg ES, Energy Procedia 2014; 63:5938- 5958.
- Hume SA, Shah MI, Lombardo G, Kleppe ER. Results From CESAR1 Testing with Combined Heat and Power (CHP) Flue Gas at the CO2 Technology Centre Mongstad, TCCS-11 Trondheim Conference on CO2 Capture, Transport & Storage, Trondheim, Norway, June 21-23 2021. Pg 355 – 361
- Kvamsdal HM, Haugen G, Svendsen HF, Tobiesen A, Mangalapally H, Hartono A, Mejdell T. Modelling and simulation of the Esbjerg pilot plant using the CESAR 1 solvent. Energy Procedia 2011; 4:1644-1651.
- Accelerating Low carbon Industrial Growth through CCUS: ALIGN-CCUS, project homepage https://www.alignccus.eu/ accessed: 15.11.2021
- Benquet C, Knarvik A, Gjernes E, Hvidsten OA, Kleppe ER, Akhter S. First Process Results and Operational Experience with CESAR1 Solvent at TCM with High Capture Rates (ALIGN-CCUS Project), 15th International Conference on Greenhouse Gas Control Technologies, GHGT-15, 15th -18th March 2021 Abu Dhabi, UAE http://dx.doi.org/10.2139/ssrn.3814712
- Further Assessment of Emerging CO2 Capture Technologies for the Power Sector and their Potential to Reduce Costs IEAGHG, 2019-09
- Wiechers G, Moser P, Benquet C, Gibbons J, Monteiro J, Hartono A, Solli KA, Knuutila H. Guidelines for effective solvent management, Deliverable Nr. D1.2.6, ALIGN-CCUS.
- Hume SA, Shah MI, Lombardo G, de Cazenove T, Feste JK, Maxson A, Benquet C. Results from MEA testing at the CO2 Technology Centre Mongstad. Verification of Residual Fluid Catalytic Cracker (RFCC) baseline results, 15th International Conference on Greenhouse Gas Control Technologies, GHGT-15, 15th -18th March 2021 Abu Dhabi, UAE
- Campbell M, Akhter S, Knarvik A, Zeeshan M, Wakaa A. CESAR1 Solvent Degradation and Thermal Reclaiming Results from TCM Testing, 16th International Conference on Greenhouse Gas Control Technologies, GHGT-16, 23rd– 27th October 2022, Lyon, France.
- Moser P, Wiechers G, Schmidt S, Garcia M, Monteiro J, Goetheer E, Charalambous C, Saleh A, van der Spek M, Garcia
- S. ALIGN-CCUS: Results of the 18-Month Test with Aqueous AMP/PZ Solvent at the Pilot Plant at Niederaussem – Solvent Management, Emissions and Dynamic Behaviour (February 8, 2021). Proceedings of the 15th Greenhouse Gas Control Technologies Conference 15-18 March 2021, http://dx.doi.org/10.2139/ssrn.3812132
- Mangalapally HP, Hans H. Pilot plant experiments for post combustion carbon dioxide capture by reactive absorption with novel solvents. Energy Procedia 2011; 4:1-8.
- Rabensteiner, Markus, et al. “Pilot plant study of aqueous solution of piperazine activated 2-amino-2-methyl-1-propanol for post combustion carbon dioxide capture.” International Journal of Greenhouse Gas Control 51 (2016): 106-117.
- Schumacher N, et al. “CESAR 1 solvent test campaign at the pilot facility at Esbjerg power station”, CESAR Project Deliverable (2010).
Results from CESAR-1 Testing with Combined Heat and Power (CHP) Flue Gas at the CO2 Technology Centre Mongstad (2021)
Scott A. Hume,2* Muhammad I. Shah,1 Gerard Lombardo,1 and Eirik Romslo Kleppe1
1Technology Centre Mongstad (TCM), 5954 Mongstad, Norway 2Electric Power Research Institute, Inc., 3420 Hillview Avenue, Palo Alto, CA 94394, USA *Corresponding author

Abstract

Technology Centre Mongstad (TCM) houses a demonstration-scale test facility for CO2 capture solvents termed the “amine plant,” where multiple test campaigns have been performed on numerous solvents that the owners of TCM, TCM DA, have conducted since its inauguration in 2012. The large number of public, industrial, research, and academic participants involved in these campaigns have enriched the projects and ensured that the significant results serve a broad audience. The main objective of these campaigns was to produce knowledge that can be used to reduce the cost as well as the technical, environmental, and financial risks for the commercial-scale deployment of post- combustion CO2 capture (PCC). This includes demonstration of a model-based control system, dynamic operation of the amine plant, investigation of amine aerosol emissions, and establishment of the baseline performance with monoethanolamine for residual fluid catalytic cracker (RFCC) and combined-cycle gas turbine (CCGT)-based combined-heat-and-power plant (CHP) flue gases. The RFCC flue gas is sourced from a nearby Equinor refinery that emulates coal flue gas in composition with 13%–14% vol CO2 content and the CHP flue gas represents flue gas from CCGT power plants with a 3.5% vol CO2 content. In addition to baseline testing, specific tests targeted at reducing CO2 avoided cost have also been conducted utilizing both flue gas sources. This paper focuses on the testing of the CESAR-1 solvent, a blend of 2-amino-2-methyl-1-propanol and piperazine.
The Electric Power Research Institute, Inc. (EPRI) assessed the performance of the process using an independent verification protocol (IVP) developed previously. The IVP provides a structured testing procedure for assessing the thermal and environmental performance of PCC processes under normal operating conditions. Throughout the CESAR-1 testing, TCM manually collected extractive samples from the depleted flue gas and product CO2 outlets sequentially. As part of the IVP, EPRI also assessed critical plant instrumentation at TCM for accuracy and precision error based on a comparative analysis done during testing operations and against calibration checks.
The CESAR-1 process was evaluated during 16 individual test periods over four days in June 2020. During the tests, extractive samples were taken to measure process contaminants such as aldehydes, ketones, amines, and ammonia. Sulfur oxides and nitrogen oxides were continuously monitored using Fourier-transform infrared (FTIR) analysers on the depleted flue gas and product CO2 streams. TCM has installed multiple measurements (FTIR, non-dispersive infrared sensor, and gas chromatography) of the CO2 concentration allowing comparative confirmation during the test periods. The capture rate was calculated via four methods along with evaluation of the CO2 recovery, which is indicative of the overall mass balance. The overall thermal performance (energy consumption) was assessed based on measured data taken during each of the sampling periods. The CO2 capture rate achieved during the CESAR-1 testing was 97–99%, with steam reboiler duties of 3.41–3.54 GJ/tonne CO2, and the CO2 gas mass balance closures were close to 100%. These data and the associated assessments, along with the results of TCM sampling during these tests, are presented in this paper.
1. Introduction
The Technology Centre Mongstad (TCM) is located next to the Equinor refinery in Mongstad, Norway. TCM DA is a joint venture owned by Gassnova representing the Norwegian state, Equinor, Shell, and TotalEnergies. TCM is home to one of the largest post-combustion CO2 capture (PCC) test centers in the world. This facility entered the operational phase in August 2012 and is dubbed the “amine plant.”
A unique aspect of the facility is that either a flue gas slipstream from a natural gas-fired combined-heat-and-power (CHP) plant or an equivalent volumetric flow from a residual fluid catalytic cracker (RFCC) unit can be used for CO2 capture. The CHP flue gas contains about 3.5 vol% CO2 and the RFCC flue gas contains about 13–14 vol% CO2, the latter of which is comparable to CO2 levels seen in a coal-fired flue gas. The amine plant, designed and constructed by Aker Solutions and Kværner, is a highly flexible and well-instrumented facility that can accommodate a variety of technologies with capabilities of treating flue gas streams of up to 60,000 Sm3/hr.
The plant is offered to developers of solvent-based CO2 capture technologies to test the performance of their solvent technology and to verify systems aimed to reduce the atmospheric emissions and environmental impact of solvent emissions and degradation products from these processes.
The objective of TCM DA is to test, verify, and demonstrate CO2 capture technologies suitable for deployment at full scale. A significant number of vendors, including Aker Solutions, Alstom (now GE Power), Cansolv Technologies Inc., and Carbon Clean Solutions Ltd., have already successfully tested using the TCM DA facilities to assess their CO2 capture technologies.
Multiple tests using the CESAR-1 solvent have been carried out at TCM to define the baseline performance of the solvent for defined operating conditions using CHP flue gas boosted to 5 vol% CO2 content using recycle in accordance with an independent verification protocol (IVP), which provides a structured testing procedure, developed by the Electric Power Research Institute, Inc. (EPRI) [1]. These tests are compared with prior MEA testing at TCM using the CHP flue gas without recycle at 3.5 vol%.
2. Amine Plant
The TCM 234 tonnes-CO2/day amine plant was designed to be flexible to allow testing of different configurations. The amine plant is configured to remove CO2 from a natural gas-fired combustion turbine-based CHP plant flue gas or a RFCC off-gas. The typical characteristics of these two flue gas streams are shown in Table 1.
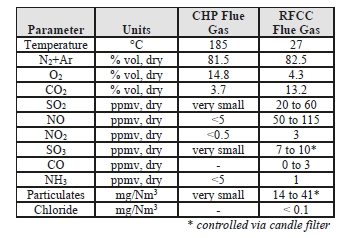
Table 1: Nominal characteristics of flue gas supplie.
For these tests, a portion of the product CO2 was recycled to the CHP flue gas inlet stream in a controlled way to maintain the incoming CO2 concentration at 5% vol, dry. A process flow diagram showing high-level equipment contained within the amine plant along with key existing instrumentation is shown in Figure 1.
Major systems include:
- An induced draft (ID) blower to overcome pressure drops and blow the flue gas through the plant with an output capacity of up to 270 mbar and 70,000 Sm3/hr.
- A direct-contact cooler (DCC) system to initially lower the temperature of and saturate the incoming flue gas by a counter-current water flow to improve the efficiency of the absorption process and provide pre- scrubbing of the flue gas. The DCC system has two individually operated packed columns for operations with the CHP flue gas and the RFCC flue gas, respectively. The DCC column designed for CHP gas operations is 3-m diameter and a total 16 m height. The section where water counter currently contacts the flue gas is 3.1 m high with Flexipac 3X structured stainless- steel packing of Koch Glitsch.
- An absorber to remove CO2 from the flue gas. The absorber has a rectangular, polypropylene-lined concrete column with a 3.55 x 2 m cross-section and a total height of 62 m. The lower regions of the tower, where the amine solution contacts the flue gas, consist of three sections of Koch-Glitsch Flexipac 2X structured stainless-steel packing of 12 m, 6 m, and 6 m of height, respectively. Water-wash systems are located in the upper region of the tower to scrub and clean the flue gas, particularly of any solvent carry over, and consist of two sections of Koch-Glitsch Flexipac 2Y HC structured stainless-steel packing, each 3 m in height. The lower water-wash section is used to cool the depleted flue gas for overall plant water balance by adjusting the temperature of the circulating water. The uppermost water-wash section was operated as an adiabatic acid-wash stage for further emission mitigation. Liquid distributors, liquid collector trays, and mesh mist eliminators (Koch-Glitsch) are located at various locations in the tower, and the final mesh mist eliminator at the top of the tower is by Sulzer. The CO2 depleted flue gas exits the absorber column to the atmosphere through a stack located at the top of the column.
- Stripper columns to recover the captured CO2 and return CO2-lean solvent to the absorber. The amine plant consists of two independent stripper columns with a common overhead condenser system. The two stripper columns are operated independently considering the CO2 content in the flue gas due to column design, hydraulics, and gas velocity effects. The smaller diameter stripper column is used when treating CHP flue gas or RFCC gas diluted with air, whereas the larger diameter column is used when treating flue gases of undiluted (i.e., higher CO2 content) RFCC gas or when operating with CHP using CO2 recycle, as is the case with these tests. The CHP stripper is 1.25 m in diameter and 28 m in height tangent-to-tangent. The RFCC stripper is 2.2 m in diameter and is also 28 m tangent-to-tangent. The lower regions of both stripper columns, where the amine solution is stripped, consist of Koch-Glitsch Flexipac 2X structured stainless-steel packing 8 m high. The upper regions of the strippers consist of a rectifying water-wash section of Koch-Glitsch Flexipac 2Y HC structured stainless-steel packing 1.6 m high. Liquid distributors, liquid collector trays, and mesh mist eliminators (all by Koch-Glitsch) are located at various locations in the strippers. Each stripper column is connected to its respective steam-heated reboiler, providing the necessary heat required for the stripping process. Both strippers circulate solvent to the reboilers by thermosiphon. The RFCC stripper also has a circulating pump to assist at low-load operation and during startup. The RFCC reboiler is a shell-and-tube arrangement and the CHP reboiler is a plate-in shell heat exchanger.
- A lean-solvent trim cooler that uses seawater to cool the lean solvent leaving the cross heat exchanger to a desired temperature before admission to the absorber column.
- A set of pumps used to move the CO2-lean and CO2-rich solvent streams between the absorber and stripper and through a cross heat exchanger to recover heat from the lean stream.
- A reflux drum, condenser, and pumps to dry the product CO2 that exits the stripper. A portion of the product CO2 can also be recycled back to the inlet of the CHP DCC to increase the concentration of the CO2 in the inlet flue gas stream when using CHP flue gas.
The TCM facility can test virtually any PCC solvent- based process as the amine plant has been designed to accommodate a variety of technologies. The facility also has excellent instrumentation and an on-site lab for detailed analysis.
An IVP was developed to be used as part of the overall performance assessment of amine-based processes and has been updated over time to apply to either CHP or RFCC operation on the TCM amine facility. The IVP is designed to provide a structured testing procedure for assessing thermal and environmental performance of PCC processes under normal operating conditions. Uncertainty for key flow measurements was carried out as part of the IVP previously [2].
3. CESAR-1 CHP Campaign Overview
The CESAR-1 solvent is a blend of 2-amino-2-methyl-1- propanol (AMP) and piperazine (PZ). CHP flue gas capture performance assessment periods were conducted in June 2020. During the testing, personnel from TCM manually collected extractive samples from the depleted gas outlet and the product CO2 line downstream of the RFCC stripper. In previous tests, this was sometimes performed by an independent testing contractor.
However, TCM’s competency related to performing this testing was deemed adequate by EPRI during prior monoethanolamine (MEA) baseline campaigns, especially since TCM is not commercially involved in the outcome and hence can be considered to be unbiased.
Data logs for all sampling periods containing pertinent flows, temperatures, pressures, and concentrations measured by permanent plant instruments were supplied by TCM for the entire test period. The sampling time periods, and sampling period designators are shown in Table 2 along with additional sampling undertaken on each day.
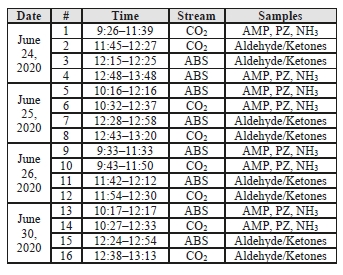
Table 2: CESAR-1 CHP sampling periods.
The plant operated in a stable condition through the entire test period, as shown in Figures 2 and 3 with controlled flue gas flow at 59,000 Sm3/h and CO2 controlled at 5 vol%, dry using recycled product gas.
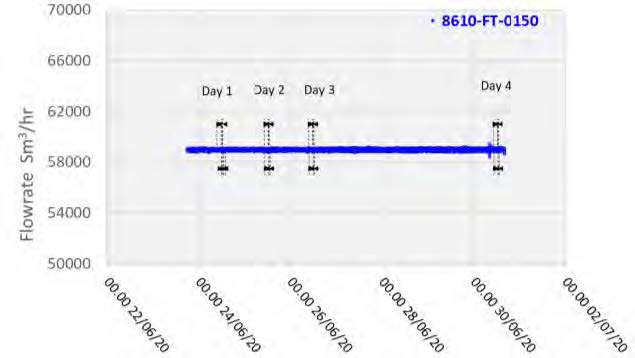
Figure 2: Flue gas flowrate through testing period.
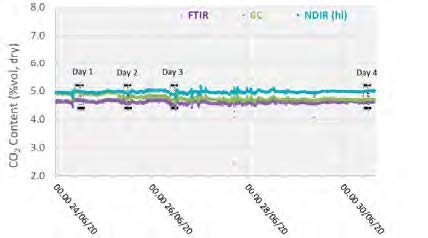
Figure 3: Inlet CO2 concentration through testing period.
3.1 CO2 Capture Efficiency and Recovery
CO2 capture efficiency can be quantified in several ways depending on how measurements have been taken and the expected accuracy of each individual measurement. Using different combinations of the measured parameters at the boundary of the process, four individual methods can be applied as detailed in Table 3.
These methods can rely on combinations of the available information to determine a capture efficiency, using the measured gas flowrates in combination with the CO2 analyzer measurements.
Method 4 simplifies the measurement uncertainty by utilizing only CO2 concentration data and making the well-founded assumption that all incoming inert gases (such as nitrogen and oxygen) will be unchanged through the absorption process. Hence, Method 4 can be used to compare against the other methods that utilize the flow measurements.
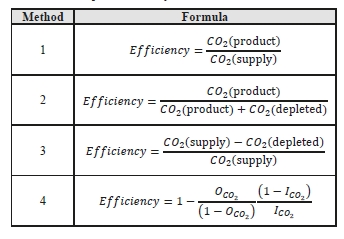
Table 3: CO2 capture efficiency calculation methods.
The “CO2 recovery” calculation is defined as the ratio of
the sum of the CO2 flow in depleted flue gas and the
product CO2 flow divided by the CO2 flow in the flue gas
supply.
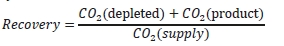
The CO2 recovery is a measure of the closure of the CO2 mass balance, being the fraction of CO2 mass flow in the
flue gas supply that is accounted for by measured CO2 mass flows in the depleted flue gas and product CO2.
Table 4 shows the four calculation methods of CO2 capture and recovery for the test periods. Note that CO2
product flow can be based on either the measured CO2 product flow or by using the difference between the nondispersive infrared-measured CO2 supply and depleted flows. CO2 capture rates calculated by all methods were in good agreement within each test period. It should be noted that Methods 3 and 4 are equivalent due to using the conserved oxygen and nitrogen method for outlet gas flow determination.
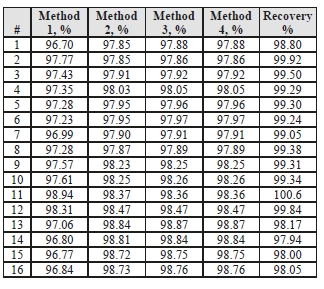
Table 4: CESAR-1 CHP sampling periods.
Regardless of the method used, the CO2 capture rate was consistently >96% as measured during all test periods. As the recovery rate was close to 100%, this implies consistency between the flue gas measurements and CO2 concentration determination at all 3 locations.
3.2 Thermal Use
The reboiler heat duty or the heat released in the reboiler is calculated as the difference between steam enthalpy at reboiler inlet and the saturated water enthalpy at the reboiler condensate temperature. The specific thermal use (STU) is then calculated by dividing the reboiler heat duty by the product CO2 flow.
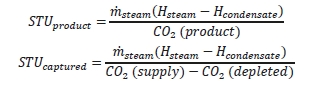
The two corresponding values for specific thermal energy consumption are shown in Table 5 and were consistent during all test periods.
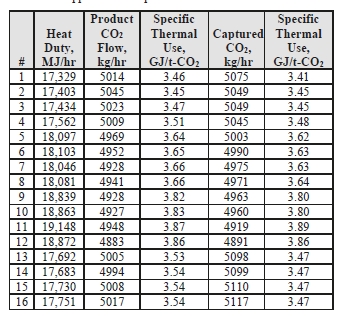
Table 5: Stripper reboiler specific thermal use.
Prior testing at TCM using conventional 5M MEA solvent with CHP flue gas (3.5 vol% CO2) at approximately an 80 tonnes-CO2/day load yielded a regeneration energy range of 3.61–3.66 GJ/t-CO2 using the product CO2 flow and 3.58–3.60 GJ/t-CO2 using the capture method, all carried out at 85% capture rate [3]. The CESAR-1 CHP tests (5 vol% CO2) achieved circa 119 tonnes-CO2/day load and achieved a regeneration energy range of 3.45–3.87 GJ/t-CO2 using product flow and 3.41–3.89 GJ/t-CO2 using the gas-side difference method.
It can be seen that the regeneration energy initially was near the bottom of the range for Tests 1 to 4 on June 24, and steadily increased in subsequent Tests 5 to 12. It was identified by TCM that excess foam formation in the stripper caused additional water condensation in the overhead stripper and an associated steam consumption increase. An antifoam agent was injected in the morning of June 30 by TCM operators with a subsequent rapid reduction in the regeneration energy measured in Tests 13 to 16, implying that the baseline CHP CESAR-1 regeneration energy is more in the range of 3.45–3.54 GJ/t-CO2 using product flow and 3.41–3.48 GJ/t-CO2 using the gas-side difference method when foaming is absent.
Importantly, the capture rate is 98% for these tests, far higher than the 85% capture rate for the MEA baseline tests, showing CESAR-1 solvent performs well at high capture rates as the regeneration energy is lower than MEA (when foaming was controlled) despite a capture rate of nearly 100%.
Recent testing at the Niederaussem pilot plant showed CESAR-1 solvent at 98% capture rate required only 3.22 GJ/tonne regeneration energy, however the inlet CO2 concentration was 15.2% vol, dry as the flue gas source is from coal combustion [4]. Although the lower CO2 concentration during these tests resulted in higher regeneration energy than observed at Niederaussem, some of the difference can also be attributed to the use of the RFCC stripper for these tests that is oversized for this regeneration load, operating at only 50% capacity. The CHP stripper was not used for these tests due to a combination of the 5% vol, dry inlet CO2 concentration and the targeted 98% capture rate.
3.2 Process Contaminants
3.2.1 Aldehydes and Ketones
Formaldehyde, acetaldehyde, and acetone concentrations were determined by extractive sampling during the CESAR-1 CHP test periods. The data are shown in Table 6 for the depleted flue gas and in Table 7 for the CO2 product.
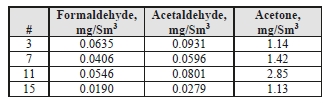
Table 6: Depleted flue gas aldehyde/ketone concentrations.
The formaldehyde levels are lower than the previous MEA CHP baseline testing, which measured concentrations of 0.72 mg/Sm3 by an external contractor. The acetaldehyde levels are also considerably lower with CESAR-1 than the MEA CHP test samples of 16 mg/Sm3.
Acetone levels measured during the MEA tests were sufficiently low at or below the detection limit of 1 mg/Sm3, while with CESAR-1 they were measurable at between 1–3 mg/Sm3 even though the upper water wash was configured as an acid wash for these tests and not in the MEA campaign These species were also measured continuously with the Proton-Transfer-Reaction mass spectrometer (PTR-MS) that exhibits a very low detection limit capability (measuring in the ppb range). A sample of the data collected is shown in Figure 4, with higher formaldehyde levels (700–800 ppb) than measured by extractive samples (20–50 ppb), and comparable acetaldehyde and acetone measurements.
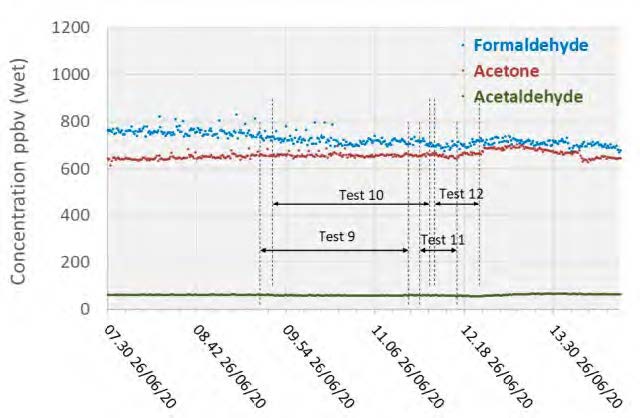
Figure 4: Depleted flue gas PTR-MS aldehyde and ketone measurements.
For the CO2 product, the formaldehyde levels detected were 2–4x higher than the manual-sampled measurements during the MEA CHP baseline campaign (0.14 mg/Sm3) and the acetaldehyde levels were considerably lower than the previous level of 150 mg/Sm3 measured for MEA.
Unlike the MEA tests, acetone was easily detected in the CO2 product for CESAR-1, whereas in the previous MEA baseline all measurements taken were below the detection limit of 0.9 mg/Sm3.
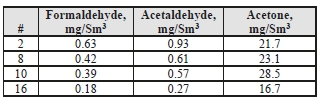
Table 7: Product CO2 aldehyde/ketone concentrations.
The concentration of the depleted flue gas will be impacted by the CO2 recycle stream, passing a portion of the contaminants shown back to the absorber inlet. With the exception of acetone, these components in the flue gas for the CESAR-1 testing were significantly lower than previous MEA measurements. This in turn suggests that these components are not significant degradation compounds from AMP and PZ, or that these solvents were not degraded to the same condition as for MEA.
3.2.2 Ammonia and Solvent Components
TCM measured concentrations of solvent components (AMP and PZ) along with ammonia during the CESAR- 1 testing. Results of these manually extracted samples are shown in Table 8.
The solvent components of CESAR-1 appear to show higher vapor pressure than is associated with MEA solvent, which was previously measured by an external contractor at 0.006 mg/Sm3 during testing on CHP flue gas. PZ was barely detected, only showing up in Test 4, which shows that perhaps a longer extraction sample period would help to improve determination of this species at the ppb level. Ammonia levels are far lower than the previous MEA CHP tests results, measured at 13 mg/Sm3, suggesting that ammonia does not represent a significant degradation product of CESAR-1.
With the exception of the first test, the AMP measurements were lower than the extractive samples. However, both strategies were likely near their method detection limits as levels were measured below 20 ppb in all cases. PZ was not detected by the PTR-MS instrument and hence was not included in Figure 5, however Acetonitrile was detected at 0.1 ppmv.
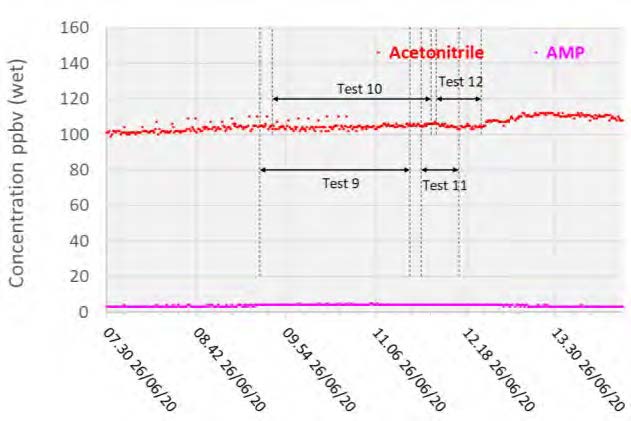
Figure 5: Depleted flue gas PTR-MS solvent measurements.
Extractive solvent and ammonia samples were taken from the CO2 product, and the results are shown in Table 9.
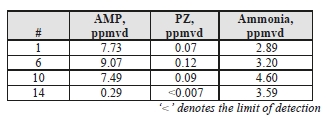
Table 9: Product CO2 ammonia and solvent component concentrations.
Similar to the depleted flue gas measurements, the AMP measurements were higher than the equivalent MEA samples, at 0.076 mg/Sm3, and up to 2 orders of magnitude higher for AMP. PZ was detected in 3 of the 4 samples, but was present at very low concentrations.
Although ammonia desorption into the product gas is 2 orders of magnitude higher than the depleted flue gas levels, this is 4 times lower than the ammonia detected from the MEA CHP tests at 16 mg/Sm3.
3.3.3 SO2 and NOx
The TCM Fourier-transform infrared units installed for the flue gas supply and the depleted flue gas were configured to measure SO2, NO, and NO2 concentrations. The reported data are shown in Figures 6 and 7.
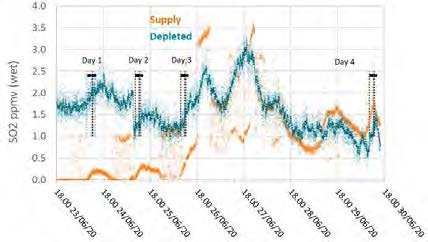
Figure 6: Supply and depleted flue gas SO2 measurements throughout the test period.
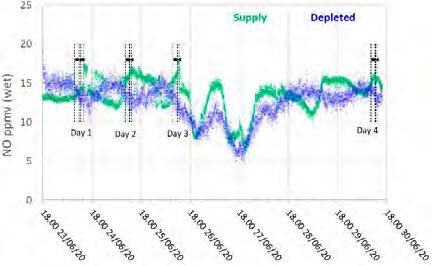
Figure 7: Supply and depleted flue gas NO measurements throughout the test period.
During previous MEA testing, SO2 levels leaving the absorber were consistently lower than the inlet measurement, likely due to absorption. This doesn’t appear to be the case for CESAR-1 solvent, though the inconsistency in the incoming flue gas data doesn’t allow a strong relationship to be established. Therefore, there was no way to determine SO2 absorption rates for this solvent from the tests.
The NO data shows similar levels at the inlet and outlet, indicating minimal absorption into the CESAR-1 solvent. While absorbed NO2 is known to contribute to the formation of nitrosamines in some solvents, the NO2 data for the depleted gas were not recorded for these test periods. The average measured values in the flue gas for both NO and SO2 leaving the absorber are shown in Table 10.
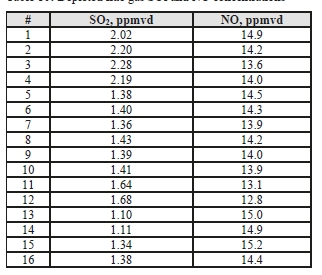
Table 10: Depleted flue gas SO2 and NO concentrations.
4. Conclusions
CESAR-1 solvent was tested at the TCM amine plant over 16 individual tests, during which extractive samples were taken, an overall summary of the tests is given in Table 11.
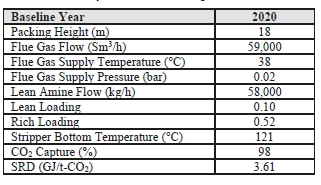
Table 11: Summary of CESAR-1 testing.
The plant was operated at 119 tonnes/day of CO2 production with capture rates of 96–99%, exhibiting a near 100% mass balance.
Foaming was identified as causing stripper performance issues; however, when foaming was controlled, the regeneration energy for CESAR-1 solvent was 3.41–3.54 GJ/t-CO2, lower than prior baseline testing of MEA at a lower capture rate of 85% at 3.58–3.66 GJ/t-CO2.
Degradation products including formaldehyde, acetaldehyde and ammonia, were measured at lower levels for CESAR-1 solvent with acid water wash compared to results from MEA testing. As the solvent is a blend of AMP and PZ, both species were sampled showing higher levels of AMP than was measured for MEA and PZ being barely detectable due to the low vapor pressure of PZ.
5. Acknowledgements
The authors gratefully acknowledge the staff of TCM DA, Gassnova, Equinor, Shell, and Total for their contribution and work at the TCM DA facility.
The authors also gratefully acknowledge Gassnova, Equinor, Shell, and TotalEnergies as the owners of TCM DA for their financial support and contributions.
6. References
- Thimsen et al., Energy Procedia 63, 5938–5958; 2014.
- Flue Gas Composition and Energy & Mass Balance Accuracy Studies at CO2 Technology Centre Mongstad (TCM DA): Results Related to the Amine Capture Unit.” K-E Frøysa and R. Sakariassen.CRM-12-F14016-RA-2: 7 June 2012
- Faramarzi et al., Energy Procedia 114, 1128–1145; 2017.
- Moser et al., ALIGN-CCUS: Results of the 18- Month Test with Aqueous AMP/PZ Solvent at the Pilot Plant at Niederaussem – Solvent Management, Emissions and Dynamic Behavior. Proceedings of the 15th Greenhouse Gas Control Technologies Conference 15-18 March 2021, http://dx.doi.org/10.2139/ssrn.3812132
Results from MEA testing at the CO2 Technology Centre Mongstad. Verification of Residual Fluid Catalytic Cracker (RFCC) baseline results (2021)
Scott A. Humeb*,Muhammad I. Shaha, Gerard Lombardoa, Thomas de Cazenovea, Jane K. Festea, Andrew Maxsonb, and Christophe Benqueta
aTechnology Centre Mongstad (TCM), 5954 Mongstad, Norway bElectric Power Research Institute, Inc, 3420 Hillview Avenue, Palo Alto, CA 94394, USA

Abstract

Technology Center Mongstad (TCM) houses a pilot-scale test facility for CO2 capture solvents termed the “amine plant”, where multiple test campaigns have been performed on monoethanolamine (MEA). The third MEA test campaign (MEA-3) was conducted in June 2017 and several subsequent tests on MEA (MEA-4 and MEA-5) were performed, through October 2018. MEA- 3, MEA-4, and MEA-5 have been the most significant collaborative test campaigns that the owners of TCM, TCM DA, have conducted since its inauguration in 2012. The large number of public, industrial, research, and academic participants involved in these campaigns have enriched the projects and ensured that the significant results will serve a broad audience. The main objective of these campaigns was to produce knowledge that can be used to reduce the cost as well as the technical, environmental, and financial risks for the commercial-scale deployment of post-combustion CO2 capture (PCC). This includes demonstration of a model-based control system, dynamic operation of the amine plant, investigating amine aerosol emissions, establishment of residual fluid catalytic cracker (RFCC)—a flue gas emanating from a nearby Equinor refinery that emulates coal in composition—baseline performance with MEA, and specific tests targeted at reducing CO2 avoided cost. Through the campaigns, both flue gas sources currently available to TCM were used, including the RFCC gas as well as flue gas from a nearby combined-cycle gas turbine (CCGT)-based combined-heat-and-power plant (CHP) that operates off of natural gas.
The Electric Power Research Institute, Inc. (EPRI) assessed the performance of the MEA-based process using an independent verification protocol (IVP) previously developed for the CHP flue gas [1]. The IVP provides a structured testing procedure for assessing the thermal and environmental performance of PCC processes under normal operating conditions. Based on this, methodology results were presented by Faramarzi et al [2]. The IVP was updated for use with the RFCC as this gas contains 13– 14 vol% CO2 content by volume whereas the CHP flue gas has 3.5 vol% CO2 content. Throughout the RFCC testing, TCM DA manually collected extractive samples from the depleted flue gas and product CO2 outlets sequentially. As part of the IVP, EPRI also assessed critical plant instrumentation at TCM for accuracy and precision error based on a comparative analysis done during testing operations and against calibration checks.
The MEA baseline process was evaluated during thirteen individual test periods over four days in May 2018. During the tests, extractive samples were taken to measure process contaminants such as aldehydes, ketones, amines, and ammonia. Sulfur oxides and nitrogen oxides were continuously monitored using Fourier-transform infrared (FTIR) analysers on the depleted flue gas and product CO2 streams. TCM DA has installed multiple measurements of the CO2 concentration (FTIR, non-dispersive infrared sensor, and gas chromatography) allowing comparative confirmation during the test periods. The capture rate was calculated via four methods. CO2 recovery (overall mass balance) was evaluated and the thermal performance (energy consumption) was assessed based on measured data taken during the tests. The CO2 capture rate achieved during the MEA RFCC testing was close to 90%, with steam reboiler duties of 3.43–3.51 GJ/tonne CO2, and the CO2 gas mass balance closures were close to 100%.
These data and the associated assessments, along with the results of TCM DA sampling during these tests, will be presented in this paper and will provide a new baseline case for 30 wt% MEA solvent in higher concentration flue gas capture cases. Based on this, TCM will now have two baselines covering flue gases with 3.5 vol% CO2 (Faramarzi et al.) and with 13–14 vol% CO2 (this project).
1. Introduction
The Technology Centre Mongstad (TCM) is located next to the Equinor refinery in Mongstad, Norway. TCM DA is a joint venture owned by Gassnova representing the Norwegian state, Equinor, Shell, and Total. The test facility, dubbed the “amine plant”, run by TCM DA entered the operational phase in August 2012 and is one of the largest post-combustion CO2 capture (PCC) test centres in the world. A unique aspect of the facility is that either a flue gas slipstream from a natural gas-fired combined-heat-and-power (CHP) plant or an equivalent volumetric flow from a residual fluid catalytic cracker (RFCC) unit can be used for CO2 capture. The CHP flue gas contains about 3.5 vol% CO2 and the RFCC flue gas contains about 13–14 vol% CO2, the latter of which is comparable to CO2 levels seen in coal-fired flue gas. The amine plant, designed and constructed by Aker Solutions and Kværner, is a highly flexible and well-instrumented unit that can accommodate a variety of technologies with capabilities of treating flue gas streams of up to 60,000 standard cubic meters per hour. The plant is offered to developers of solvent-based CO2 capture technologies to test the performance of their solvent technology and to verify technologies aimed to reduce the atmospheric emissions and environmental impact of amines and amine-based degradation products from solvent- based CO2 capture processes. The objective of TCM DA is to test, verify, and demonstrate CO2 capture technologies suitable for deployment at full-scale. A significant number of vendors, including Aker Solutions, Alstom (now GE Power), Cansolv Technologies Inc., Carbon Clean Solutions Ltd., Fluor and Ion Engineering have already successfully used the TCM DA facilities to verify their CO2 capture technologies.
Multiple tests using monoethanolamine (MEA) have been carried out at TCM to define the baseline performance of the solvent for defined operating conditions using flue gas from the CHP at 3.5 vol% CO2 content [2]. More recently, the MEA solvent has been tested with the higher CO2 concentrations from the RFCC flue gas to develop a new baseline for the amine plant, in accordance with an independent verification protocol (IVP), which provides a structured testing procedure, developed by the Electric Power Research Institute, Inc. (EPRI) [1].
2. Amine plant
The schematic of the TCM DA amine plant when treating the CHP flue gas is shown in Figure 1.
3. IVP overview
The roles and responsibilities of the organizations that conducted the IVP are:
- TCM DA is the prime on the project and its personnel organized the field testing during the test period. They also operated the plant throughout all baseline testing
- EPRI were contracted by TCM DA to develop the IVP during previous MEA baseline testing. EPRI was on site during MEA baseline testing on RFCC flue gas to observe the conduct of the tests and the associated manual extractive sampling. EPRI received the data for the RFCC tests from TCM DA for analysis.
4. Test campaign
The testing of the MEA solvent at TCM was carried out using RFCC flue gas that has CO2 concentrations of typical coal flue gases. Prior testing with MEA using CHP flue gas was conducted in September 2015. The test periods identified for the RFCC flue gas operation, shown in Table 1, reflect the extractive sampling periods carried out on 28–31 May 2018.
During the testing, personnel from TCM DA manually collected extractive samples from the depleted gas outlet and product CO2 line downstream of the RFCC stripper. In previous tests, this was sometimes performed by an independent testing contractor. However, TCM DA’s competency related to performing this testing was deemed adequate by EPRI, especially since TCM DA is not commercially involved in the outcome and hence can be considered to be unbiased.
Data logs for all sampling periods containing pertinent flows, temperatures, pressures, and concentrations measured by permanent plant instruments were supplied by TCM DA for the entire test period.
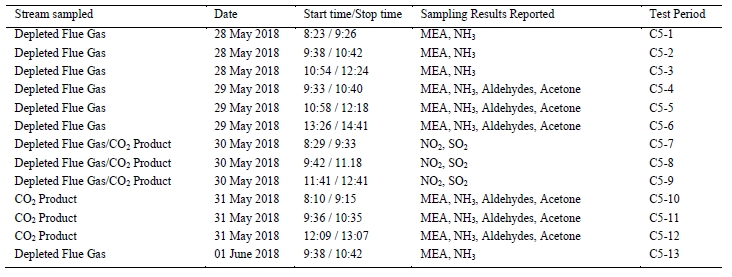
Table 1. Summary of the test periods.
5. Instrument assessment
To determine the process plant performance, a key component is the quality of the instrumentation installed for measuring the respective compositions and flow rates. Instrumentation quality is determined using two parameters:
- Accuracy/bias: This represents the difference between the instrument reading (or average of a set of readings under unchanging process conditions) being assessed and the true value of the parameter being measured. Appropriate determination of the “true value” must be achieved by simultaneous measurement of the parameter using a reference method or instrument with calibration that can be traced to primary standards.
- Precision: A determination of the variability of the instrument reading when stream conditions are known to be steady state. Precision is therefore a measure of the random error associated with the measurement.
These measurement errors can be combined to assess the aggregate uncertainty in each measurement. In the absence of a calibration against primary standards for the entire measurement range needed, the uncertainty published by the instrument supplier represents only the precision error.
When the process parameter being measured does not change, precision is a measure of repeatability. In real plant situations, when attempting to operate at steady-state conditions, often process parameters (flow, pressure, and temperature) do vary over the measurement period. Thus, measurements over long periods of time (greater than process time constants) will also include an error term related to process uncertainty.
5.1 Gas-phase compositions
TCM DA has 3 independent Fourier-transform infrared (FTIR) units (Finetech Anafin 2000 and a pair of Gasmet FCX units), facilitating dedicated and continuous FTIR measurements at the absorber inlet, outlet, and CO2 product gas streams. The CO2 concentration at the inlet and outlet of the absorber column is also determined by two non- dispersive infrared (NDIR) units at each location, one set to high range (% vol) and one to low range (ppmv) on a dry- gas basis. A dedicated trace O2 instrument (Teledyne Instruments 3001) is installed to quantify O2 content of the product CO2 as this is typically at ppm levels in this stream. A Siemens Maxum Edition II gas chromatograph (GC) unit is also installed, which is capable of measuring the CO2, O2, and nitrogen content at all three locations in a near- simultaneous fashion.
During the May to June 2018 operations, TCM DA utilized the installed FTIR systems, NDIR analysers, and GC unit to monitor the incoming flue gas and depleted flue gas, as shown in Figure 2 and Figure 3. It should be noted that the low-range NDIR units could not be used for RFCC testing as the inlet flue gas measurement range is 0–5 vol% (dry) and the outlet depleted flue gas range is 0–1 vol% (dry), both of which are below the gas concentrations measured during these tests.
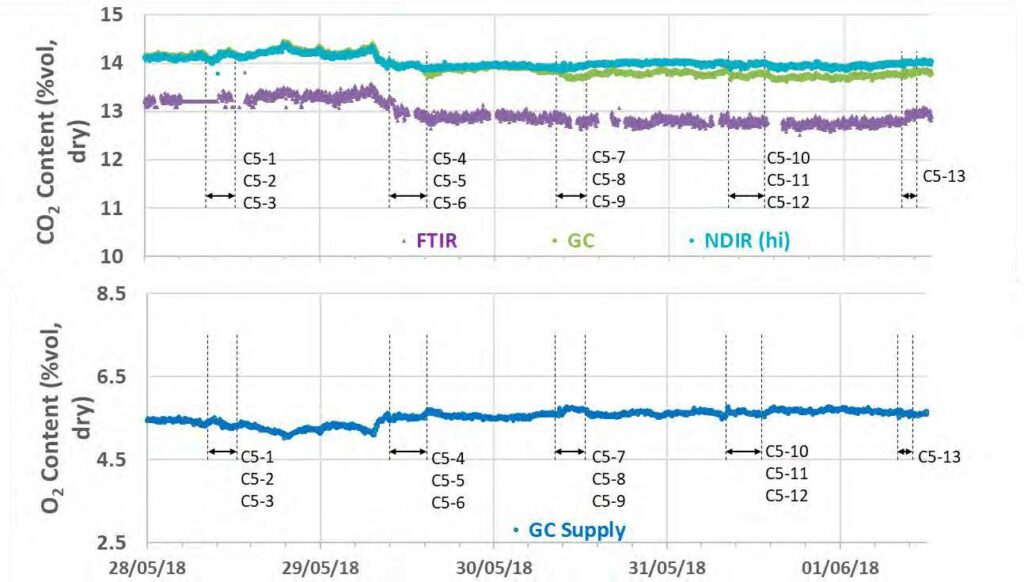
Figure 2. RFCC flue gas supply CO2 and O2 data for all analysers.
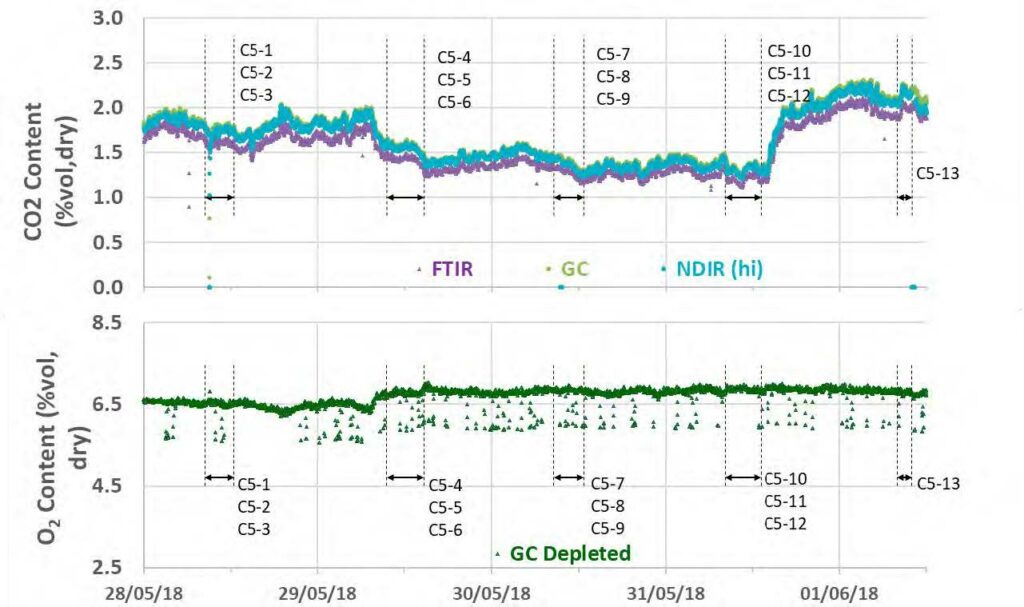
Figure 3. Depleted flue gas CO2 and O2 data.
- Figure 2 displays the RFCC flue gas supply CO2 and O2 concentration data over the test campaign. There is good agreement between CO2 NDIR and the GC CO2 measurements (<0.2% point difference). The TCM FTIR CO2 was biased 1% point lower than the other two instruments.
- Figure 3 displays the depleted flue gas CO2 and O2 concentration data over the test campaign. The data from all of the TCM instruments show close tracking together, suggesting that the process CO2 concentration had a degree of variability (±0.2% vol) during that operating period.
- The O2 content of the product CO2 was low in the range of 1–2 ppmv, as shown in Figure 4. For the purposes of calculating CO2 removal and recovery, it is assumed here that the product CO2 stream is saturated with water at the measured temperature and pressure and contains the small trace quantities of O2 and N2 measured. The balance is presumed to be CO2.
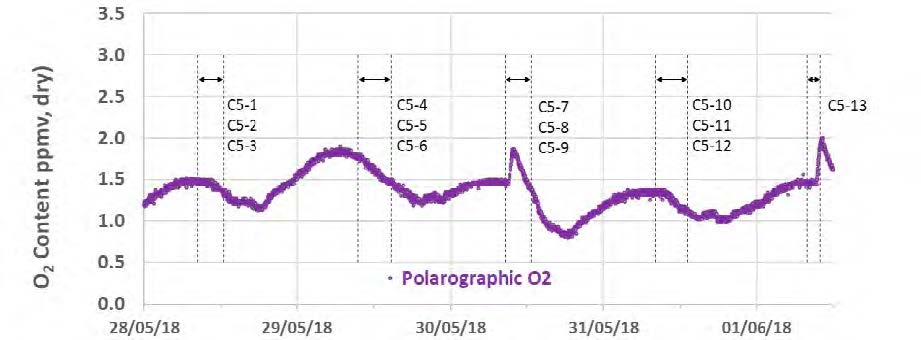
Figure 4. Product gas O2 data.
5.2 Gas-phase flow rates
Supply flue gas, depleted flue gas, and the CO2 product streams were determined by TCM DA plant instrumentation for continuous measurement of the flow rates. In particular, the TCM DA amine plant is well instrumented for determining the RFCC flue gas supply flow rate, with several of the flow meters positioned in series.
- The RFCC flue gas supply flow is measured by two independent multi-pitot-tube flow meter instruments, (FIC-0024 and FT-2039), which are characterized in Table 2. The data from these flow meters are shown in Figure 5. All flow rates are at the defined standard conditions of 15°C and 101.3 kPa. The RFCC flue gas flow was very steady over the entire test period. The test period flow averages used for all calculations are the data reported by the multi-pitot-tube (FIC-0024) as this had a lower precision error.
Table 2. Key flow instrumentations (precision uncertainties are based on internal assessments by TCM DA).
- The depleted flue gas flow is measured by a single multi-pitot tube flow meter (FT-2431), whose characteristics are listed in Table 2. As observed in prior campaigns, the measured flow has significant transients that are not correlated with any process parameter. Subsequently, the depleted flue gas flow rate was calculated assuming that all O2 and N2 entering with the flue gas supply leaves in the depleted flue gas. The performance data reported here use such a calculation for depleted flue gas flow rate.
- The product CO2 flow measured by the vortex flow meter (FT-0010) is the primary flow meter used by TCM DA operators, the characteristics for which are listed in Table 2. Additionally, a Coriolis flow meter is installed (FT-2215), however this instrument has not undergone an accuracy study and so is not used for primary assessment. The data from both flow meters are shown Figure 6. The product CO2 flow was relatively steady over all periods.
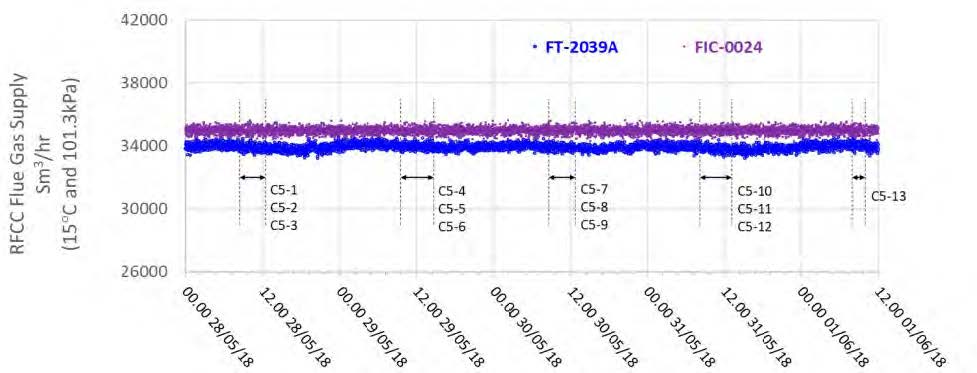
Figure 5. Flue gas supply flow measurements for RFCC testing May-June 2018.
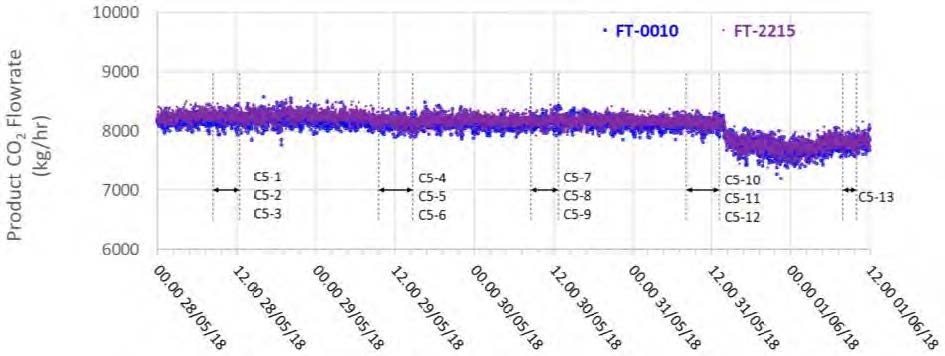
Figure 6. Product flue gas flow rate measurements for RFCC testing May-June 2018.
5.3 Steam and condensate flow rates
The TCM DA amine plant receives high-pressure (HP) superheated steam from the neighbouring refinery at a pressure of approximately 30 bar, and a temperature of between 240°C to 310°C. A schematic of the system supplying steam to the stripper reboiler is shown in Figure 7.
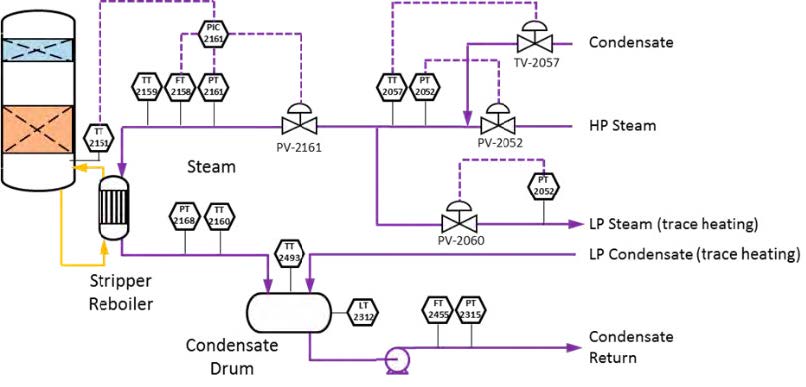
Figure 7. RFCC stripper reboiler steam supply flow schematic.
The HP steam is throttled near the stripper reboiler to a pressure of approximately 5 bar before being desuperheated with condensate. Following condensation in the stripper reboiler, the steam condensate collects in a receiving vessel before being returned to the refinery.
Steam heat tracing is facilitated using a small amount of medium-pressure steam that is reduced to a lower pressure prior to use. The resultant low-pressure (LP) steam condensate is returned to the same receiver as the stripper reboiler condensate. For thermal energy consumption assessment, the key parameter of interest is the steam flow to the reboiler, shown in Figure 8.
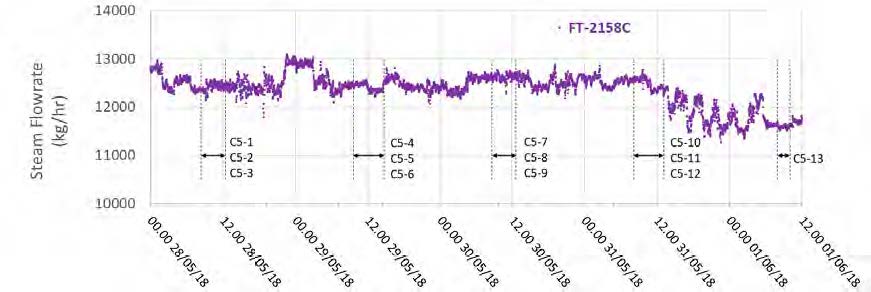
Figure 8. RFCC reboiler steam flow.
6. Results and discussion
6.1 CO2 capture efficiency and recovery
The CO2 capture efficiency was calculated using the four methods (Methods 1–4) via the formulas detailed in Table 4. In addition, CO2 recovery is the fraction of CO2 mass flow in the flue gas supply that is accounted for by measured CO2 mass flows in the depleted flue gas and product CO2—it is a measure of the degree to which the CO2 mass balance is closed.
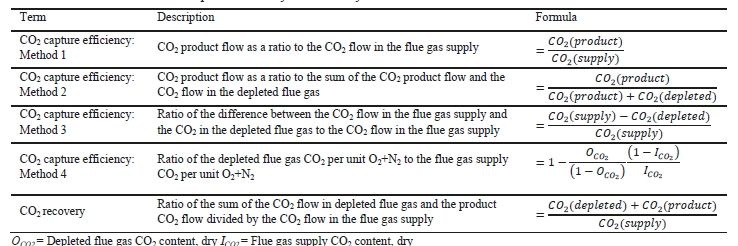
Table 3. CO2 capture efficiency and recovery calculation methods.
As the depleted flue gas flow measurement was not reliable, it was assumed that the oxygen and nitrogen entering the absorber with the flue gas leaves in the depleted flue gas. The saturated water content of the depleted flue gas was calculated using its temperature and pressure. The CO2 flow out of the absorber was calculated using the concentration of CO2 in the depleted flue gas and the calculated mass flowrate. These are essentially the same assumptions as those used for Method 4, which is independent of flowrate and uses a concentration-only approach. Subsequently, Method 3 and Method 4 calculations result in identical CO2 capture rates.
The CO2 recovery was then estimated using the calculated flow of depleted flue gas. The calculated CO2 capture efficiency and recovery are presented in Table 4. For all test periods, the calculated CO2 captures were quite steady near the 90% capture target and the CO2 recovery was consistently in the range of 101–104%. Test C5-13 was targeted at the lower 85% capture rate.
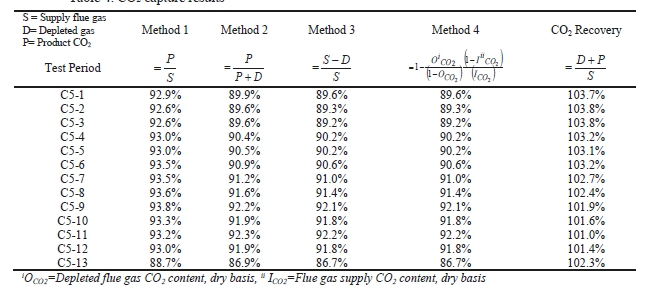
Table 4. CO2 capture results.
The uncertainty calculations and results from calculation Methods 1–3 are shown in Table 5. The following assumptions were used:
- Flow metering uncertainties were calculated by TCM DA for the indicated flow meters based on the specification of the instrument [3].
- Concentration uncertainties for the flue gas flows are those detailed in Table 2.
- Concentration uncertainty for the product CO2 is assumed to be 1% to allow for CO2 content as low as 99%.
- CO2 capture of 90% is representative of that measured during all test periods.
- The uncertainty in CO2 capture (ECO2) is almost entirely due to uncertainty in CO2 content of the RFCC flue gas supply for the assigned total (low) flow uncertainties. The CO2 capture uncertainty is relatively insensitive to uncertainties in the CO2 contents of both the product CO2 and depleted flue gases.
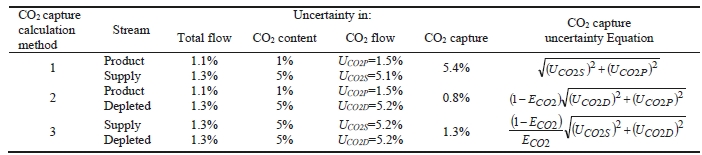
6.2 Thermal energy consumption
The reboiler thermal duty was calculated as the difference between steam enthalpy at the reboiler inlet temperature and pressure and the saturation enthalpy of water at the reboiler condensate temperature. The specific thermal use (STU) was obtained by dividing the reboiler duty by the product CO2 flow. The CO2 product flow was either based on the measured CO2 product flow (P) or on the difference between the NDIR-measured CO2 supply flow and the estimated CO2 depleted flow (S – D). The two corresponding values for STU are shown in Table 6. The results for STU were very consistent during all test periods. The product flow measurements (P) were consistently higher than the using the gas-side difference method (S – D) method, resulting in lower STU values.
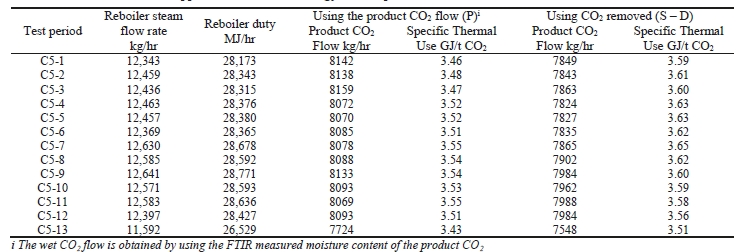
Table 6. Stripper reboiler thermal energy consumption.
Prior testing with CHP flue gas at approximately an 80 tonnes per day load yielded a regeneration energy range of 3.61–3.66 GJ/tonne CO2 using the product CO2 flow (P) and 3.58–3.60 GJ/tonne CO2 using the gas-side difference method (S – D) [2]. The RFCC tests achieved circa 190 tonnes per day load and delivered an average regeneration energy of 3.51 GJ/tonne CO2 using product flow (P) and 3.60 GJ/tonne CO2 using gas-side difference method (S – D). This represents a 2% reduction in regeneration energy, likely due to the higher inlet CO2 levels.
6.3 Gas-phase contaminants
Formaldehyde, acetaldehyde, and acetone concentrations were determined by extractive sampling of the depleted flue gas at the absorber outlet during the RFCC test periods as shown in Table 7. The formaldehyde levels are lower than the previous MEA baseline testing values of 720 μg/Sm3 and 40 g/hr, which were done by an independent, contractor (FORCE Technology) using brought-in instruments. The acetaldehyde levels are considerably lower than the 2015 MEA test samples of 16 mg/Sm3 and 840 g/h emission levels measured by FORCE Technology. However, acetone levels measured were sufficiently low to be at or below the detection limit of the analysis performed by SINTEF, a similar result to the previous MEA test [2].
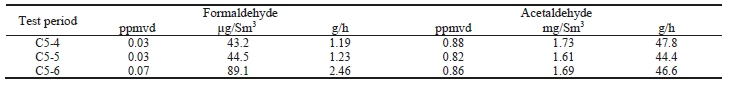
For the CO2 product, shown in Table 8, the formaldehyde levels detected are lower than the manually sampled measurements during the 2015 CHP baseline campaign of 140 μg/Sm3 and the acetaldehyde levels are considerably lower than the previous level of 150 mg/Sm3. The 2015 MEA baseline measurements were closer to the levels measured in 2014, where formaldehyde was detected at 190 μg/Sm3. Although acetone was detected in the CO2 product, these measurements were at or below the detection limit, whereas in the previous MEA baseline all measurements taken were below the detection limit.
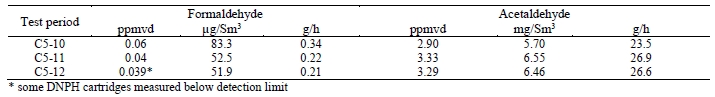
Table 8. Product gas aldehyde concentrations.
TCM DA measured concentrations of MEA and ammonia at the absorber outlet during the RFCC test periods C5-1 to C5-6 and for C5-13 following a modification to the process operating conditions to a lower capture rate target. The results of these manually extracted samples are shown in Table 9.
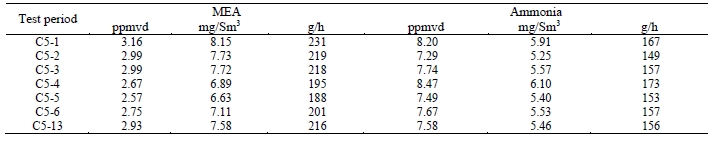
Table 9. Depleted flue gas stream ammonia and amine concentrations and mass rates as phase contaminants.
The levels detected here for MEA were consistent between samples and are far higher than observed by FORCE Technology during the 2015 CHP gas testing (0.006 mg/Sm3). Although the levels measured here are higher, previous EPRI experience with coal-derived flue gases has observed comparable single-digit ppm levels of amine at the depleted flue gas location when aerosol levels are low or zero, which TCM DA had achieved using the Brownian demister filter upstream of the absorber. Ammonia levels are lower than the previous CHP tests results, measured at 13 mg/Sm3. However, it is unknown how degraded the solvent was during these tests in comparison with the previous campaign. TCM DA measured concentrations of MEA and ammonia in the CO2 product gas during the RFCC test periods C5-10 to C5-12. MEA was not detected, and levels of ammonia were substantially lower at 0.05- 0.08mg/Sm3 than the prior CHP test results [2]. While extractive samples of SOx and NOx were not carried out during this test campaign, the FTIR was used to track the relative levels of each component. The RFCC flue gas was expected to have 20–60 ppm (vol, dry) of SO2 from previous tests, but this couldn’t be reliably verified during these tests as the FTIR instrument did not deliver a reliable measurement. The outlet measurements were more consistent, as shown in Table 10. The consistent <1 ppm vol measurement of SO2 suggests absorption by the MEA solvent.
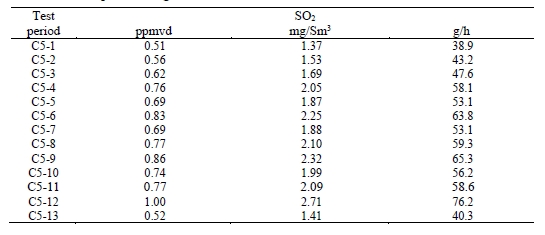
Table 10. Depleted flue gas SO2 concentrations and mass rates.
The NOx quantities passing through the absorber were effectively unchanged, showing only the concentrating effect of the CO2 removal from the flue gas, where more than 10% of the flue gas volume is removed. As the amine plant operated continuously capturing 90% throughout the test period, the FTIR-measured NOx data show a consistent increase in concentration at the outlet of the absorber as shown in Figure 9.
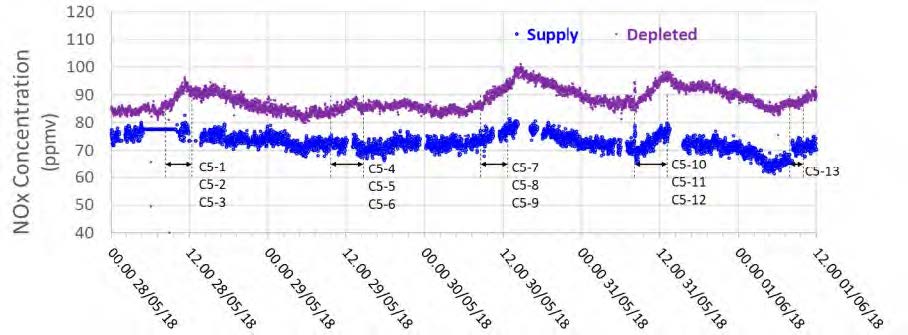
Figure 9. Absorber inlet and outlet NOx values.
6.4 New baseline for solvent performance testing
Table 11 presents a portion of the MEA test data obtained at the TCM DA amine plant. Based on these data, a new baseline was established for higher concentration flue gas CO2 capture.
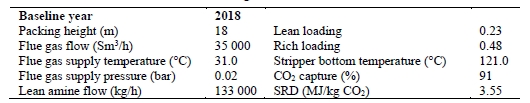
Table 11. Results of RFCC baseline testing in 2018.
This baseline represents the performance of the TCM amine plant close to the plant nominal capacity using 5M MEA solvent with higher CO2 concentration flue gas (13–14 vol%), typical of coal-based thermal plants. Alongside the prior baseline work carried out in 2015 for flue gas with CO2 concentrations from natural gas-fired CHP units (3–4 vol%) [2], this new baseline will serve as the performance benchmark for other amines tested at the TCM DA amine plant when using RFCC flue gas.
Acknowledgements
The authors gratefully acknowledge the staff of TCM DA, Gassnova, Equinor, Shell, and Total for their contribution and work at the TCM DA facility. The authors also gratefully acknowledge Gassnova, Equinor, Shell, and Total as the owners of TCM DA for their financial support and contributions.
References
- Thimsen et al. Results from MEA testing at the CO2 Technology Centre Mongstad. Part I: Post-Combustion CO2 capture testing methodology; Energy Procedia 63, 5938–5958; 2014.
- Faramarzi et al. Results from MEA testing at the CO2 Technology Centre Mongstad: Verification of baseline results in 2015; Energy Procedia 114, 1128–1145; 2017.
- Flue Gas Composition and Energy & Mass Balance Accuracy Studies at CO2 Technology Centre Mongstad (TCM DA): Results Related to the Amine Capture Unit.” K-E Frøysa and R. Sakariassen. CRM-12-F14016-RA-2: 7 June 2012
Results from MEA testing at the CO2 Technology Centre Mongstad: Verification of baseline results in 2015 (2016)
Leila Faramarzia,b,*, David Thimsenc, Scott Humec, Andrew Maxonc, Guillaume Watsond, Pedersen Sa, Erik Gjernese, Berit F. Foståsb, Gerard Lombardoe, Toine Centsf, Anne Kolstad Morkena,b, Muhammad Ismail Shaha,e, Thomas de Cazenovea, Espen Steinseth Hamborga,b
aCO2 Technology Centre Mongstad (TCM DA), 5954 Mongstad, Norway bStatoil ASA, P.O. Box 8500, 4035 Stavanger, Norway cElectric Power Research Institute, Inc., 3420 Hillview Avenue, Palo Alto, CA 34304, USA dShell Global Solutions International B.V., PO Box 663, 2501CR The Hague, The Netherlands eGassnova SF, Dokkvegen 10, 3920 Porsgrunn, Norway fSasol Technology, P.O. Box 5486, Johannesburg 2000, South Africa *Corresponding author

Abstract

In 2015, the CO2 Technology Center Mongstad (TCM DA) operated a post-combustion CO2 capture test campaign using aqueous monoethanolamine solvent at 30 weight%. The main objective was to demonstrate and document the performance of the TCM DA amine plant located in Mongstad, Norway.
During the treatment of flue gas from the natural gas-fired combined heat and power plant at Mongstad, a revised baseline was established for the TCM DA amine plant in accordance to the verification protocol developed by the Electrical Research Institute, Inc. This paper presents the revised baseline, which can be considered as a reference case for the solvent-based CO2 capture processes applied to natural gas-based flue gases.
1. Introduction
The CO2 Technology Centre Mongstad (TCM) is located next to the Statoil refinery in Mongstad, Norway. TCM DA is a joint venture set up by Gassnova representing the Norwegian state, Statoil, Shell, and Sasol. The facility run by TCM DA entered the operational phase in August 2012 and is one of the largest post-combustion CO2 capture (PCC) test centres in the world. A unique aspect of the facility is that either a flue gas slipstream from a natural gas- based combined heat and power (CHP) plant or an equivalent volumetric flow from a residual fluid catalytic cracker (RFCC) unit can be used for CO2 capture. The CHP flue gas contains about 3.5% CO2 and the RFCC flue gas contains about 13-14% CO2, the latter of which is comparable to CO2 levels seen in coal-fired flue gas. One of the main test plants at TCM DA is a highly flexible and well-instrumented amine plant. The amine plant was designed and constructed by Aker Solutions and Kværner to accommodate a variety of technologies, with capabilities of treating flue gas streams of up to 60,000 standard cubic meters per hour. The plant is being offered to vendors of solvent-based CO2 capture technologies to, among others, test: (1) the performance of their solvent technology; and
(2) technologies aimed to reduce the atmospheric emissions and environmental impact of amines and amine-based degradation products from solvent-based CO2 capture processes. The objective of TCM DA is to test, verify, and demonstrate CO2 capture technologies suitable for deployment at full-scale. A significant number of vendors, Aker Solutions, Alstom (now GE Power), Cansolv Technologies Inc., and Carbon Clean Solutions Ltd. have already successfully used the TCM DA facilities to verify their CO2 capture technologies.
From 6 July to 17 October 2015 TCM DA, in collaboration with partners, operated a monoethanolamine (MEA) campaign with the main objective to document and demonstrate the amine plant performance.
TCM DA investigated the stripper performance and concluded that the use of anti-foam made it possible to utilise the full flue gas supply capacity of 60,000 standard cubic meters per hour. At the full CHP flue gas capacity, the CO2 capture rate was about 85% when MEA at 30 weight% (wt%) was used. The corresponding specific reboiler duty (SRD) was about 3.6 GJ/ton CO2. Total and CO2 mass balance closures were near 100 %. Emission levels of MEA, NH3, aldehydes, nitrosamines, nitramines, and other compounds were also measured during extractive samples for the defined time periods and were all below the permissible levels set by the Norwegian Environment Agency (Miljødirektoratet).
During the treatment of the CHP flue gas at full capacity, a revised baseline was established for the TCM DA amine plant. The revised CHP baseline was verified by the Electric Power Research Institute, Inc. (EPRI).
EPRI has developed a structured CO2 capture testing methodology for characterizing PCC processes. EPRI’s methodology is designed to provide relevant information for baselining and comparing technologies, referred to as an independent verification protocol (IVP). This methodology has been tailored to the TCM DA amine plant facility and is presented in detail elsewhere [1].
The amine plant is planned and equipped for conducting research and development activities and TCM DA has recently installed a number of additional gas-phase analysers to improve the speed and accuracy of measurements. The IVP methodology has therefore been updated by EPRI to reflect these recently installed instruments.
The revised CHP baseline was verified by EPRI, following their requirements including the use of third-party gas phase and emission measurements done by FORCE Technology. FORCE Technology performed comprehensive measurements on flow rates, temperatures, and compositions on the absorber inlet, the absorber outlet (depleted flue gas), and the stripper outlet.
This paper will present the revised baseline for the TCM DA amine plant, in accordance to the IVP developed by EPRI.
2. Amine plant
The schematic of the TCM DA amine plant when treating the CHP flue gas is shown in Figure 1.
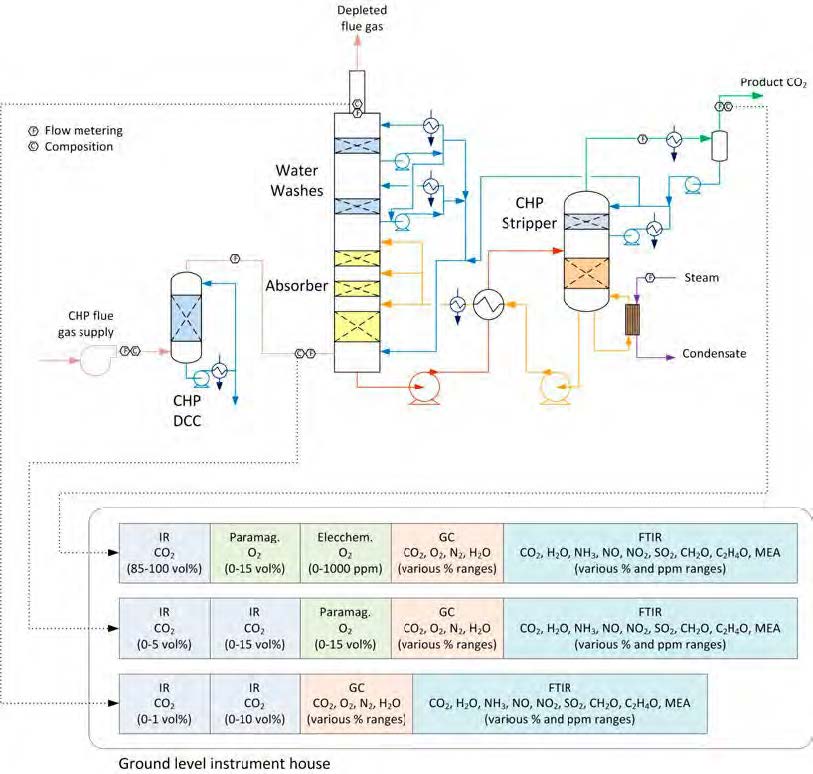
Figure 1. The TCM DA amine plant when treating the CHP flue gas.
The nominal CHP flue gas characteristics along with the existing instrumentations are specified elsewhere [2].
The main systems in the plant are also explained in detail in a previously published paper [1].
3. IVP project overview
The roles and responsibilities of the organizations that conducted the current IVP project are as follows;
- TCM DA is the project owner and organized the field testing during the test period. The test program for the baseline testing was developed by the owners of TCM DA. TCM DA personnel operated the plant throughout the testing and collected lean and rich liquid samples for laboratory analysis during the test period.
- FORCE Technology was contracted by TCM DA to collect and analyse samples from the CHP flue gas supply, depleted flue gas, and product CO2 streams. Two crews from FORCE Technology conducted the sampling sequentially with a single set of continuous emissions monitors (CEMs). FORCE Technology also collected gas samples for off-site analysis of particulate, SO2, SO3, amine, and degraded amine components.
- Laborelec carried out particulate concentration and size distribution measurements during the baselining period. Laborelec characterized the size of and the number of particles formed at different points through the absorber tower by using an electrical low-pressure impactor (ELPI+) device.
- EPRI was contracted by TCM DA to apply the IVP during the MEA baseline testing. Two EPRI engineers were on-site during the testing to observe the conduct of the tests. EPRI also led analyzing the results from the IVP project.
4. IVP
4.1 Approach
A detailed description of the IVP approach was previously reported [1]. A summary of the approach is provided here.
The purpose of the IVP is to measure and report key performance indices of the PCC process (those indices critical to up-scaling the process). Key performance indices (dependent parameters) include CO2 capture, CO2 production, emission, utility usage (steam, power and cooling), and trace constituents of the depleted flue gas and product CO2. The key performance indices depend on a number of independent parameters including: the overall process design, physical characteristics (and operating conditions) of process equipment, flue gas supply conditions and flow rate, lean and rich solutions conditions and flow rate, and stripper pressure.
Many of the dependent parameters can be modeled using commonly available chemical engineering computer process modeling tools. Field measurement of these key performance indices (along with the uncertainty in the measurements) can be used to calibrate the computer process models. Other dependent parameters (such as trace components in the depleted flue gas and product CO2) are difficult to model with currently available tools. Field measurements of these parameters will serve as primary data for up-scaling process designs.
The IVP approach to field performance testing is generally consistent with the approach taken by others for performance testing of a number of power processes [3]. The IVP specifies procedures for collecting composition, temperature, pressure, and flow data at TCM DA sufficient to calculate and report key performance indices and the corresponding numerical uncertainty in the values reported. Industry-accepted standard reference test methods are specified for the collection of composition, temperature, pressure, and flow data. Procedures for reducing the data are also specified. The IVP focuses on campaign-style testing in which days are dedicated to testing at previously selected optimum process operating conditions, but the IVP principles can also guide parametric testing undertaken to identify optimum process conditions.
4.2 MEA 2015 test campaign conduct
The second campaign of base-case testing of the performance of the TCM DA amine plant using a nominal 30% MEA as the solvent was conducted the week of 7 September 2015 after approximately eight weeks of operating the amine plant with the 30 wt% MEA solution. The plant was operated at steady state throughout the week.
FORCE Technology was on-site to manually collect contemporaneous samples from the flue gas supply, depleted flue gas, and product CO2. Laborelec was also on-site to manually collect samples for particulate and aerosol size distribution analysis at different locations through the absorber tower.
During all sampling periods the following data were collected:
- CO, CO2, NOX, O2, SO2, and N2 (by difference) concentrations in volume percent (vol%)
- Flow rate, pressure, and temperature.
The sampling time periods and sampling period designators are shown in Table 1 along with additional sampling undertaken on each day. Data logs for all sampling periods containing pertinent flows, temperatures, pressures, and concentrations measured by permanent plant instruments were supplied by TCM DA.
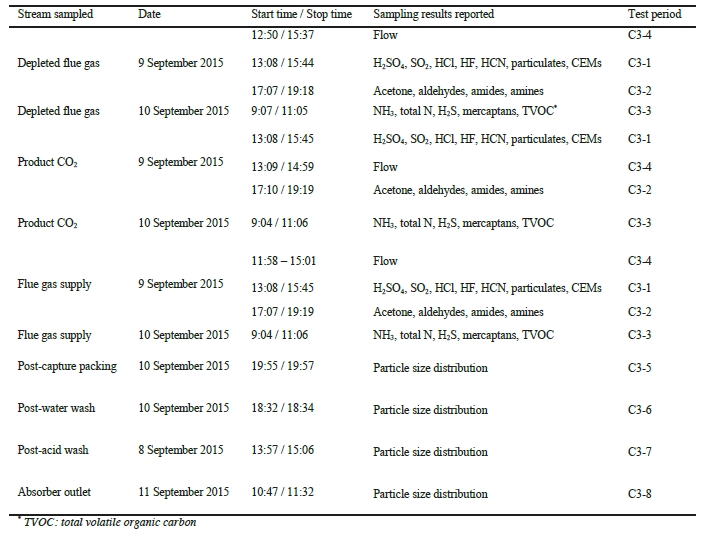
Table 1. FORCE Technology and Laborelec sampling periods.
5. Instrument assessment
An important component in the determination of process plant performance is the quality of the instrumentation installed for measuring the respective compositions and flow rates. Two measures of instrumentation quality are:
- Accuracy/bias: This represents the difference between the instrument reading (or average of a set of readings under unchanging process conditions) being assessed and the true value of the parameter being measured. Appropriate determination of the “true value” must be achieved by simultaneous measurement of the parameter using a reference method or instrument with calibration that can be traced to primary standards.
- Precision: A determination of the variability of the instrument reading when stream conditions are known to be steady state. Precision is therefore a measure of the random error associated with the measurement.
These measurement errors can be combined to assess the aggregate uncertainty in a given measurement. In the absence of a calibration against primary standards for the entire measurement range needed, the uncertainty published by the instrument supplier represents only the precision error.
When the process parameter being measured does not change, precision is a measure of repeatability. In real plant situations, it is often the case that the process parameters (flow, pressure, and temperature) do vary over the measurement period. Thus, measurements over long periods of time (greater than process time constants) will also include an error term related to process uncertainty.
5.1 Gas phase compositions
In the first baseline MEA in 2014, the CO2 and O2 content of the flue gas supply, depleted flue gas, and CO2 product stream were routinely determined by a single Fourier Transform Infrared (FTIR) instrument (Applied Instrument Technologies and Finetech, model: Anafin 2000) along with an O2 instrument (Siemens, model: Oxymat 6). Since these instruments were shared between the sampling points, a sampling system was installed to extract from the various single points as given by Thimsen et al. [1]. The sample was continuously drawn by a selection system serving the analysers and was diverted to the common analysers in a 90-minute cycle; i.e., the analyser cycles between flue gas supply for 15 minutes, depleted flue gas for 30 minutes, and CO2 product stream for 15 minutes, and an additional 30 minutes for purging operations.
Following the first MEA baseline campaign, TCM DA has since installed a number of additions to the gas measurement systems to improve the speed and accuracy of the measurements and widen the breadth of measurement techniques. To complement the original FTIR unit, two new additional Gasmet FTIR units (model: FCX) were installed, facilitating dedicated and continuous FTIR measurements at all three locations. Additionally, the CO2 concentration at the inlet and outlet of the absorber column was also determined by two non-dispersive infrared (NDIR) units (Siemens, model: Ultramat 6) at each location, one set to high range (vol%) and one low range (ppmv) on a dry-gas basis. A trace O2 instrument [Teledyne Instruments 3001] was installed to quantify O2 content of the product CO2. The system has been further complemented with a new Siemens Maxum Edition II gas chromatograph (GC) unit that is capable of measuring the CO2, O2, and nitrogen content at all three locations in a near-simultaneous fashion.
During the September 2015 operations, FORCE Technology carried out simultaneous analysis on three process streams (flue gas supply, depleted flue gas, and CO2 product stream). Comparison of the TCM DA values determined by the FTIR systems (after converting to dry basis assuming saturation at the measured pressure and temperature), NDIR analysers, and GC with the FORCE Technology data are given in Figure 2 and Figure 3. Details include:
- Figure 2 displays the CHP flue gas supply CO2 and O2 concentration data over the test campaign. There is good agreement between the FORCE Technology CO2 NDIR and the TCM GC CO2 measurements (<0.5% point difference) with the two TCM NDIR units showing a similar offset of 2% of the measured value (<0.08 vol%). The TCM FTIR CO2 average values compare well with the FORCE Technology measurements, however the instantaneous measurements showed significant scatter from the mean value (7% spread, representing ±0.3 vol%). The TCM FTIR O2 measurements agree more closely (less than 0.5 vol% dry O2) than the GC, which is over 1 vol% dry O2 higher in all measurement points. On the morning of 10 September 2015, the second O2 measurement period carried out by FORCE Technology has an overall similar offset as observed on 9 September 2015 following a morning calibration of the instrument.
- Figure 3 displays the depleted flue gas CO2 and O2 concentration data over the test campaign. The data from all of the TCM instruments closely track together, suggesting that the process CO2 concentration had a degree of variability (±0.2 vol%) during that operating period. The FORCE Technology measurements showed more variability than the TCM instruments. Data from all four TCM instruments are consistently higher than the FORCE Technology data by 10% to 25% (FTIR is the closest). The consistency in this bias, especially between the TCM NDIR and FORCE Technology NDIR instruments suggests either a difference in the calibrations of the respective instruments during the FORCE Technology campaign or, possibly, an anomaly in the FORCE Technology sampling system that diluted the sample with ambient air. It is also important to note that the FORCE Technology measurements of depleted flue gas CO2 were at or below the stated limit of detection of 0.5 vol%, although the NDIR was calibrated using “low-range” calibration gases and values down to 0.3 vol% were reported to a single significant figure to reflect the increased uncertainty of the measurements at these low levels.
- The product CO2 composition data reported by FORCE Technology had an O2 content of between 5-12 ppmv, far lower than the 1-2 vol% reported by FORCE Technology during the MEA campaign in February 2014 [4], which was thought to be contaminated by air in-leakage and subsequently disqualified. The TCM GC instrument measured nitrogen in the product with an average of 180 ppmv. For the purposes of calculating CO2 removal and recovery, it is assumed here that the product CO2 stream is saturated with water at the measured temperature and pressure and contains the small trace quantities of O2 and N2 measured. The balance is presumed to be CO2.
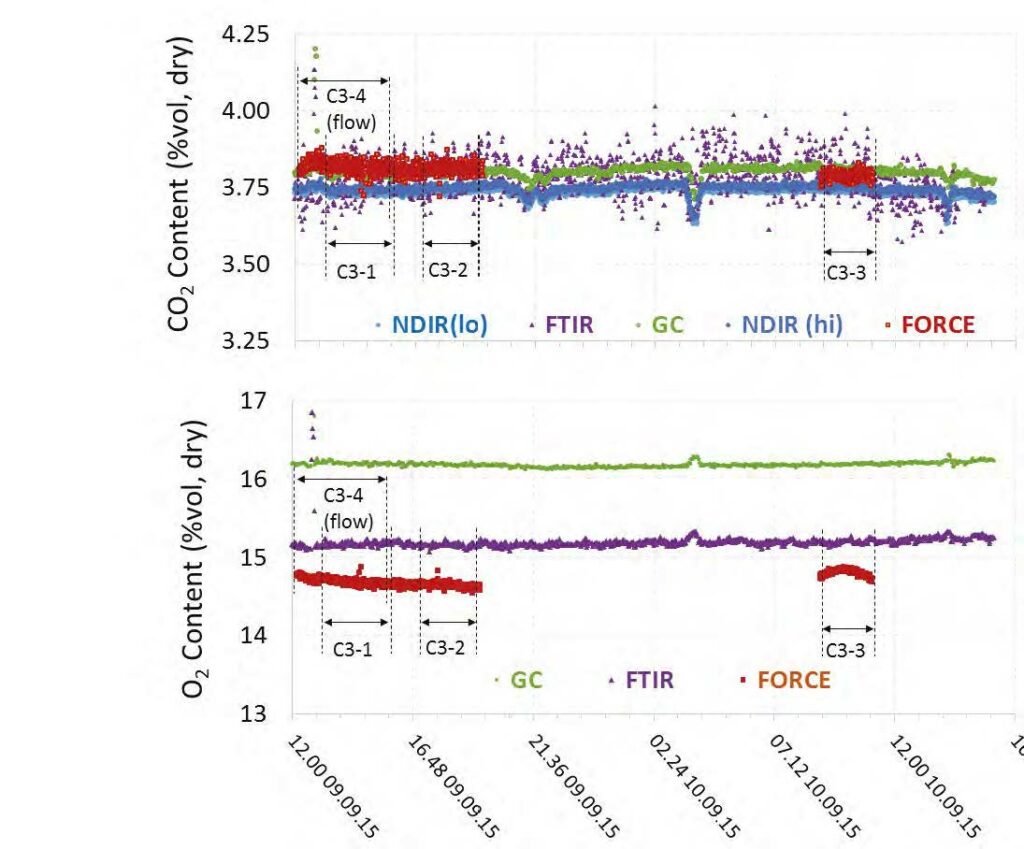
Figure 2. CHP flue gas supply CO2 and O2 data for all analysers. Data collected by FORCE Technology on 9 and 10 September 2015 are also shown.
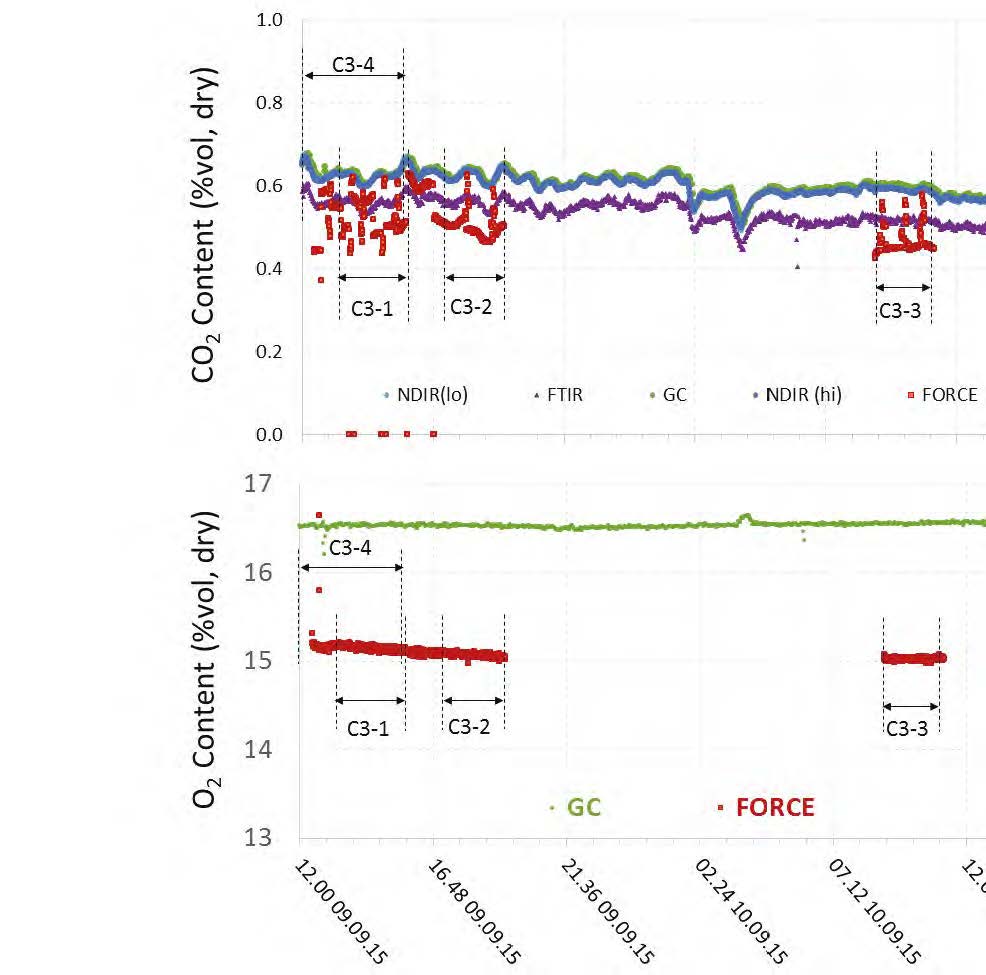
Figure 3. Depleted flue gas CO2 and O2 data. Data collected by FORCE Technology on 9 and 10 September 2015 are also shown.
5.2 Gas phase flow rates
Continuous measurement of the flow rates of the supply flue gas, depleted flue gas, and CO2 product stream were determined by TCM DA plant instrumentation. In particular, the TCM DA amine plant facility is well instrumented for determining the flue gas supply flow rate, with several different types of flow meters positioned in series.
During the base-case operations, pitot-tube traversing of the supply flue gas, depleted flue gas, and CO2 product stream was carried out by FORCE Technology to determine the flow rates, the results of which are compared to plant instrumentation measurements below:
- The CHP flue gas supply flow is measured by two independent instruments, an ultrasonic flow meter (FT-0150) and a multi-pitot-tube flow meter (FIC-0124), which are characterized in Table 2. The data from these flow meters are shown in Figure 4. All flow rates are at defined standard conditions of 15 °C and 101.3 kPa. The CHP flue gas flow was very steady over the test period on 9 September 2015 when FORCE Technology made independent measurements of flow as indicated in Figure 4. The difference between the values measured by FORCE Technology and that measured by the plant instruments is between 2–6%, well within the reported uncertainty in the FORCE Technology measurement of 10% The test period flow averages used for all calculations are the data reported by the ultrasonic flow meter (FT-0150).
- The depleted flue gas flow is measured by a single multi-pitot tube flow meter (FT-2431), whose characteristics are listed in Table 2. The measured flow had a higher degree of variability than the inlet CHP measurement (spread of 5.9% versus 0.7% for FT-0150) and also has significant transients that are not correlated with any process parameter. The data are, however, fairly consistent over the period during which FORCE Technology made independent measurements on 9 September 2015 so a comparison is possible. The individual FORCE Technology measurements average to 55,900±10% Sm3/hr, dry (101.3 kPa, 15°C) at this location. The average flow over the same time period reported by the plant flow meter is 54,200 Sm3/hr, well within the 10% uncertainty in the FORCE Technology measurement. Nevertheless, the questions associated with this measurement are sufficient to choose to calculate the depleted flue gas flow rate assuming that all O2 and N2 entering with the flue gas supply leave in the depleted flue gas. The performance data reported here use such a calculation of depleted flue gas flow rate.
- The product CO2 flow measured by the vortex flow meter (FT-0010) is the primary flow meter used by TCM operators, whose characteristics are listed in Table 2. The data from this flow meter are shown in Figure 5. The product CO2 flow was relatively steady over the test period. FORCE Technology made independent measurements of flow on 9 September 2015 as indicated in Figure 5. The difference between the value measured by FORCE Technology and that measured by the plant instruments is approximately 7.5%, within the measurement uncertainty reported by FORCE Technology of 10%.

Table 2. Key flow instrumentations. Precision uncertainties are based on internal assessments by TCM DA.
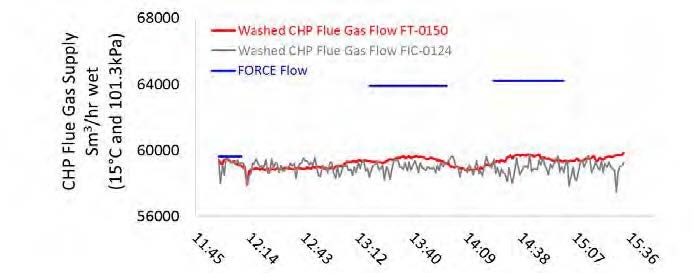
Figure 4. CHP flue gas supply flow measurements measured on 9th September 2016.
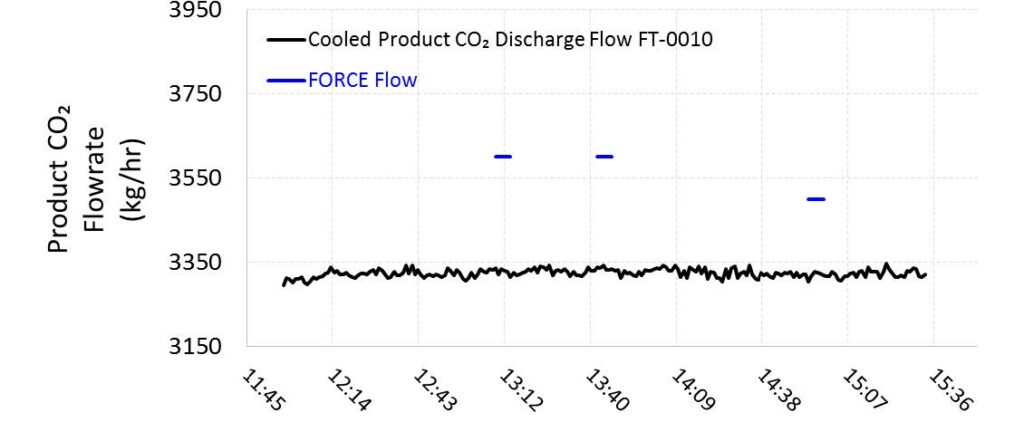
Figure 5. Product CO2 flow rate and test period averages measured on 9 September 2016.
5.3 Steam and condensate flow rates
The TCM DA amine plant receives high-pressure (HP) superheated steam from the neighbouring refinery at a pressure of approximately 30 bars and a temperature of between 240°C to 310°C. The HP steam is throttled near the stripper reboiler to a pressure of approximately 5 bar before being desuperheated with condensate. Following condensation in the stripper reboiler, the steam condensate collects in a receiving vessel before being returned to the refinery. Steam heat tracing is facilitated using a small amount of medium-pressure (MP) steam that is reduced to a lower pressure prior to use. The resultant low-pressure (LP) steam condensate is returned to the same receiver as the stripper reboiler condensate. A schematic of the system supplying steam to the stripper reboiler is shown in Figure 6. For thermal energy consumption assessment, the key parameter of interest is the steam flow to the reboiler. The HP condensate flow returned to the refinery can be assessed as a check on this parameter. The condensate return flow should be the sum of the reboiler steam flow and any condensate flow produced in steam heat tracing. Figure 7 shows these two parameters. As a result of higher ambient temperatures experienced in September 2015 the average condensate flow measurement (FT-2455) was either at or slightly lower than the steam flow measurement (FT- 2386). (During the first MEA baseline testing in January 2014, condensate measurements exceeded the steam flow measurement due to the contribution of trace heating).
Figure 6. Stripper reboiler steam supply flow schematic.
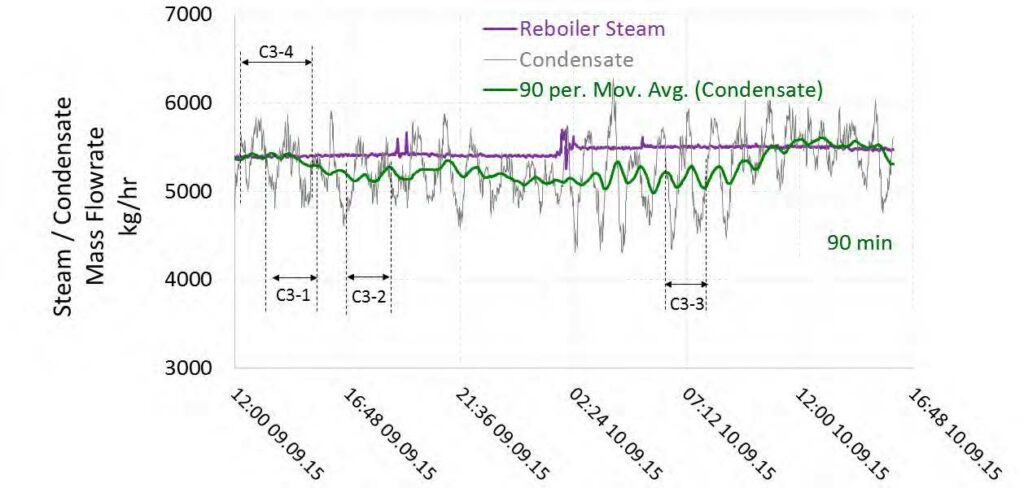
Figure 7. Reboiler steam flow and HP condensate return flow.
6. Results and discussions
6.1 CO2 capture efficiency and recovery
The CO2 capture efficiency was calculated using the four methods (Methods 1–4) shown in Table 8 in Appendix A. CO2 recovery is the fraction of CO2 mass flow in the flue gas supply that is accounted for by measured CO2 mass flows in the depleted flue gas and product CO2; it is a measure of the degree to which the CO2 mass balance is closed. The formula to calculate the amount of CO2 recovery from the flue gas supply is also given in Table 8 in Appendix A.
The depleted flue gas flow measurement was not reliable and therefore it was calculated. It was assumed that the oxygen and nitrogen entering the absorber with the flue gas leave in the depleted flue gas. The saturated water content of the depleted flue gas was calculated using its temperature and pressure. The CO2 flow out of the absorber was calculated using the concentration of CO2 in the depleted flue gas. These are essentially the same assumptions as those used for Method 4. Therefore, Method 3 and Method 4 calculations result in identical CO2 capture rates. The CO2 recovery was then estimated using the calculated flow of depleted flue gas. The calculated CO2 capture efficiency and recovery are presented in Table 3. For all test periods, the calculated CO2 capture was quite steady and the CO2 recovery was about 98–99%.
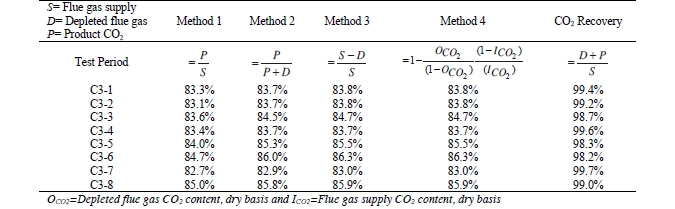
Table 3. CO2 capture results.
The uncertainty calculations and results from each calculation method are shown in Table 4. The following assumptions were used:
- Flow metering uncertainties were calculated by TCM DA for the indicated flow meters based on the specification of the instrument.
- Concentration uncertainties for the flue gas flows are those described in Section 5.2.
- Concentration uncertainty for the product CO2 is assumed to be 1% to allow for actual CO2 content as low as 99%.
- CO2 capture of 85% is representative of that measured during all test periods.
- The uncertainty in CO2 capture is almost entirely due to uncertainty in CO2 content of the CHP flue gas supply for the assigned total (low) flow uncertainties. The CO2 capture uncertainty is relatively insensitive to uncertainties both in the CO2 contents of both the product CO2 and the depleted flue gas.
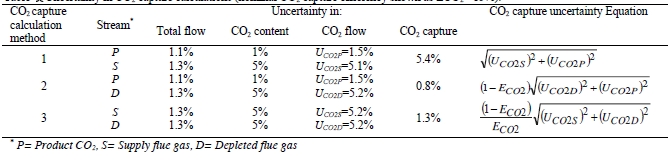
Table 4. Uncertainty in CO2 capture calculations (nominal CO2 capture efficiency shown as ECO2 =85%).
6.2 Thermal energy consumption
The reboiler thermal duty was calculated as the difference between steam enthalpy at the reboiler inlet temperature and pressure and the saturation enthalpy of water at the reboiler condensate temperature. The specific thermal duty (SRD) was obtained by dividing the reboiler duty by the product CO2 flow. The CO2 product flow was either based on the measured CO2 product flow (P) or on the difference between the NDIR-measured CO2 supply flow and the estimated CO2 depleted flow (S-D). The two corresponding values for SRD are shown in Table 5. The results for SRD were very consistent during all test periods.
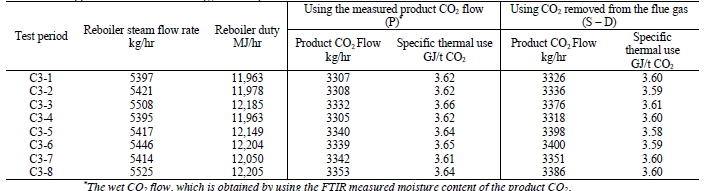
Table 5. Stripper reboiler thermal energy consumption.
6.3 Gas phase contaminants
FORCE Technology measured the gas phase concentration of the compounds listed below in the three gas streams. The data are shown in Table 9-11 in Appendix B.
- SO2 was simultaneously measured in the three gas streams during test period C3-1. A modest amount of SO2 was present in the flue gas supply. No SO2 entering the absorber in the flue gas supplied left the plant in either the depleted flue gas or product CO2 streams.
- NOX concentrations and mass flows were measured in the three gas streams during test period C3-1. NOx concentrations were below the detectable limit during all test periods.
- Acetone, formaldehyde, and acetaldehyde concentrations and mass flow rates were measured during test period C3-2. The aldehydes in the depleted flue gas and product CO2 do did exist in the supplied flue gas and were, presumably, produced in the absorption process. Acetone was not detected in any gas stream. FORCE Technology‘s measurements for the acetaldehyde concentrations were not successful and therefore the values measured by TCM DA is shown in Table 10 in Appendix B.
- Amines/Amides concentrations and mass rates were measured during test period C3-2. None of the compounds were detected in the CHP flue gas supply. The only compounds detected in the depleted flue gas and product CO2 were MEA and methylamine. Traces of ethylamine, dimethylamine, and diethylamine were detected in the depleted flue gas only. Amides were below the detection limits.
- H2SO4 concentration was measured in the three gas streams as aggregate sulfate (reported as H2SO4 equivalent) during test period C3-1. The concentration of H2SO4 was below the respective detection limits.
- Particulates were measured during test period C3-1. The total amount of particulates in the CHP flue gas supply is very low. The amount of particulates in the three gas streams was below the detection limit.
- Ammonia was simultaneously measured in the three gas streams during test period C3-3. Measurable amounts of ammonia were found in the depleted flue gas and in the product CO2. Ammonia was not detected in the CHP flue gas supply suggesting it resulted from MEA degradation during the process.
- TVOC was measured during test period C3-3. Measurable amounts of TVOC were detected in the product CO2. The CHP flue gas supply does not contain any TVOC and presumably, it resulted from MEA degradation during the CO2 capture process.
6.4 Laborelec particle measurements
Laborelec carried out particle size testing using an Electrical Low Pressure Impactor (ELPI+). Four locations of the absorber tower were monitored to investigate the potential formation of particles as the depleted flue gas passes through the washing stages and demisters. The results shown in Table 6 have measurements that were near to the detection limit of the ELPI+ when inserted in the process. The ambient air measurements undertaken during these tests were higher than the process measurements by almost one order of magnitude. The measurements were three to four orders of magnitude lower than similar measurements taken on flue gas from a coal thermal plant, proving the scarcity of particles in the CHP flue gases. The small amount of particles and their small sizes remain largely unchanged as they pass through the absorber.
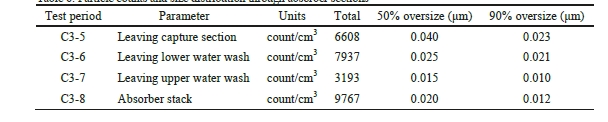
Table 6. Particle counts and size distribution through absorber sections.
6.5 New baseline for solvent performance testing
Table 7 presents a portion of the MEA test data obtained at the TCM DA amine plant. Based on these data which were obtained at about test period C3-4 when flow rates were measured, a new baseline is established. As the instrumentation of the amine plant and therefore the measurements are significantly improved since the previous MEA baseline in 2014 [4], the 2015 MEA results will set the baseline for performance benchmarking of other amines at TCM DA. The 2014 baseline is therefore considered obsolete.
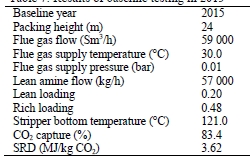
Table 7. Results of baseline testing in 2015.
Comprehensive process data for the TCM DA baseline testing in 2015 are given in Table 12, Appendix C.
7. Conclusions
The quality of the gas phase measurements at the TCM DA amine plant is significantly improved by installing new online instruments. Using the upgraded instrumentations, a new baseline for the TCM DA amine plant is established which has replaced the 2014 baseline. The new baseline is set up close to the plant nominal capacity and will serve as the performance benchmark for other amines tested at the TCM DA amine plant.
Acknowledgements
The authors gratefully acknowledge the staff of TCM DA, Gassnova, Statoil, Shell and Sasol for their contribution and work at the TCM DA facility. The authors also gratefully acknowledge Gassnova, Statoil, Shell, and Sasol as the owners of TCM DA for their financial support and contributions.
Appendix A.
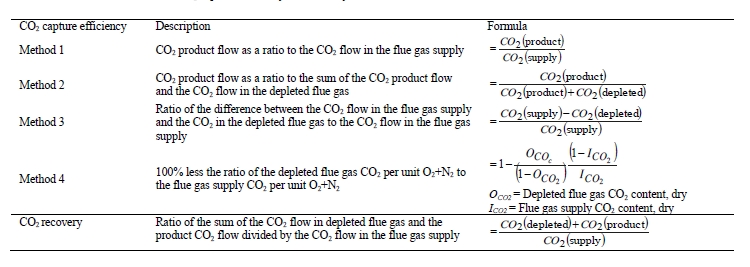
Appendix B and C
References
- Thimsen D, Maxson A, Smith V, Cents T, Falk-Pedersen O, Gorset O, Hamborg ES. Results from MEA testing at the CO2 Technology Centre Mongstad. Part I: Post-Combustion CO2 capture testing methodology Energy Procedia 63 5938-5958; 2014.
- Gjernes E, Pedersen S, Cents T, Watson G, Fostås BF, Shah MI, Lombardo G, Desvignes C, Flø NE, Morken AK, de Cazenove T, Faramarzi L, Hamborg ES. Results from 30 wt% MEA performance testing at the CO2 Technology Centre Mongstad. Energy Procedia (GHGT-13), Forthcoming 2017.
- American Society of Mechanical Engineers, New York, NY. PTC-4, Fired Steam Generators, 2008.
- Hamborg ES, Smith V, Cents T, Brigman N, Falk-Pedersen O, de Cazanove T, Chhagnlal M, Feste JK, Ullestad Ø, Ulvatn H, Gorset O, Askestad I, Gram LK, Fostås BF, Shah MI, Maxson A, Thimsen D. Results from MEA testing at the CO2 Technology Centre Mongstad. Part II: Verification of baseline results. Energy Procedia 63, 5994-6011; 2014.
Results from MEA testing at the CO2 Technology Centre Mongstad. Part I: Post-Combustion CO2 capture testing methodology (2014)
David Thimsena,*, Andrew Maxsona, Vian Smithb,c, Toine Centsb,c, Olav Falk-Pedersenb,d, Oddvar Gorsete, Espen S. Hamborgb,f
aElectric Power Research Institute, 3420 Hillview Avenue, Palo Alto, CA 94304, USA bCO2 Technology Centre Mongstad (TCM DA), 5954 Mongstad, Norway cSasol Technology, PO Box 5486, Johannesburg 2000, South Africa dGassnova SF, Dokkvegen 10 3920 Porsgrunn, Norway eAker Solutions, PO Box 222, 1326 Lysaker, Norway fStatoil ASA, P.O. Box 8500, 4035 Stavanger, Norway *Corresponding author

© 2014 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/3.0/). Peer-review under responsibility of the Organizing Committee of GHGT-12
Abstract

This paper lays out a generic CO2 capture testing methodology that has been applied at multiple sites providing details on the procedure, its key performance indices and their associated specifications, as well as the required pre-test work. Specific application of the methodology for the CO2 Technology Centre Mongstad site, a CO2 capture testing facility located in Norway that performed CO2 capture tests using MEA, is shown as an illustrative example.
1. Introduction
At the beginning of the 21st century, increasing political and technological focus is being given to minimizing carbon dioxide (CO2) emissions to the atmosphere. As the combustion of fossil fuels at large industrial facilities is a significant source of CO2 entering the atmosphere, reducing CO2 emissions from existing and new fossil-fired plants will be critical. A principal method proposed for accomplishing this reduction is to capture the CO2 produced by separating it from the flue gas into a relatively pure stream and then injecting the purified CO2 into acceptable underground geological reservoirs for long-term storage.
Currently the only CO2 capture technologies sufficiently mature to apply at full scale are temperature swing absorption (TSA) processes that remove the relatively dilute CO2 from flue gas (common in processes that use air for combustion and produce significant nitrogen that dilutes the flue gas) by chemical absorption into an alkaline solvent at low temperature. The solvent is then heated to release the CO2 in a relatively pure stream for subsequent geological storage. Aqueous amine solutions at high concentration are leading near-term solvent candidates.
The use of amines to remove CO2 from various industrial and fuel gas streams is a relatively mature technology. There is less experience using amines to remove CO2 from flue gases, which contain significant levels of oxygen. In addition, the full-scale application of amine post-combustion capture (PCC) processes for removing CO2 from flue gas would be conducted at a scale approximately an order of magnitude larger than industrial amine-based TSA processes currently deployed.
Supply of the utilities required by a TSA process (thermal, electrical, and cooling) will have a significant impact on the operations of the host plant producing the flue gas being treated. Perhaps the greatest focus of PCC development is identifying processes that minimize the use of these utilities, particularly the thermal utility.
The Electric Power Research Institute (EPRI) has developed a generic independent verification protocol (IVP) to assess the performance of amine-based TSA processes. This IVP has already been tailored to and applied during EPRI-led CO2 capture testing at the following facilities:
- AEP’s Mountaineer Plant – 20-MWe demonstration of Alstom’s chilled ammonia process during 2011–2012
- Alabama Power’s Plant Barry – 500 tonnes/day demonstration of MHI’s KM-CDR advanced amine process; testing began in 2012 and is still ongoing
- EDF’s Le Havre – 2.0-MWe demonstration of Alstom/Dow’s Advanced Amine Process (AAP) during 2014
- We Energies’ Pleasant Prairie Power Plant – 1.7-MWe demonstration of Alstom’s chilled ammonia process during 2008.
CO2 Technology Centre Mongstad (TCM DA) has installed pilot-scale amine-based TSA process equipment next to the Statoil refinery in Mongstad, Norway. The purpose of this facility is to allow vendors of suitable amine formulations and other PCC processes to test their process and collect performance data to support full-scale design and anticipate the associated performance and costs.
This work is part of a continuous effort of gaining better understanding of the performance potential of the non- proprietary aqueous MEA solvent system, conducted by TCM DA and its affiliates and owners, in order to test, verify, and demonstrate CO2 capture technologies [1, 2, 3]. As part of an overall program of CO2 capture testing, EPRI worked with TCM DA, which operates the TCM DA facility and led the testing effort, and Aker Solutions to customize the IVP for TCM DA. Details on that customization are provided within this paper.
2. Independent verification protocol purpose and scope
2.1 Amine process description
Flue gas can be supplied to the TCM DA PCC amine plant from either the on-site natural gas-fired combined heat and power (CHP) plant or from the Statoil refinery residue fluid catalytic cracker (RFCC). As the testing work that this report discusses pertains to using the CHP flue gas, details on the RFCC will not be provided here. In the CHP plant, the natural gas is combusted in a gas turbine and the flue gas content and characteristics are similar to those of a combined cycle gas turbine (CCGT) power plant.
The flow schematic for the TCM DA pilot plant when treating CHP flue gas is shown in Fig. 1 and a photo of the amine plant is shown in Fig. 2.
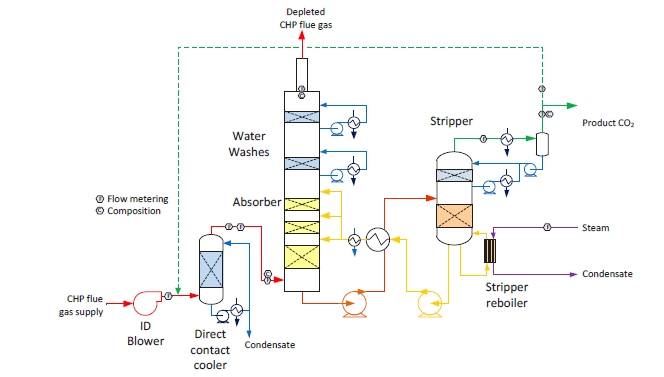
Fig. 1. Simplified flow schematic for TCM DA CO2 capture of CHP flue gas

Fig. 2. TCM DA amine plant. The direct-contact cooler is situated to the right, the concrete absorber tower in the middle, the two stripper columns to the left, and the lean vapour compressor system to the far left.
The nominal characteristics of flue gas from the CHP source both before and after the direct-contact cooler (DCC) are shown in Table 1. The CHP flue gas is typical of high excess air combustion turbine exhaust.
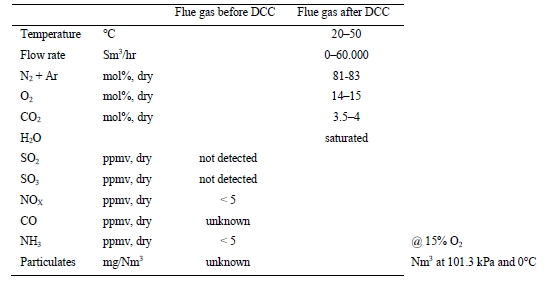
Table 1. Nominal characteristics of CHP flue gas supplied to TCM DA CO2 capture plant.
The raw flue gas may be cooled by direct contact with wash water. By these means, plant operators have the capability of controlling the temperature of the flue gas (saturated with water) delivered to the absorber.
The saturated flue gas rises in the rectangular cross-section absorber tower and comes into contact with falling lean solution in one of up to three beds of structured packing. The flue gas, depleted in CO2, then passes through up to 2 recirculating water wash stages to remove solvent vapors before being emitted to the atmosphere in a 1-meter diameter duct. The solution flow through the absorber tower is “once-through”; there is no recirculation of rich solution from the tower sump back to the top of the absorber section.
The solution rich in CO2 is pumped to the top of a stripper tower. Rich solution entering the stripper is pre-heated by exchange with hot lean solution being returned to the absorber. The falling rich solution comes into contact with rising steam/CO2. The lean solution at the bottom of the stripper is circulated through a steam-heated reboiler to provide the heat necessary to drive the endothermic CO2-releasing reactions.
The raw product CO2 leaving the stripper is cooled with recovery of condensate that is returned to the stripper as a reflux. The cooled product CO2 is vented. During CHP flue gas operations, a portion of the product CO2 can be recycled to the CHP flue gas upstream of the DCC to increase the CO2 content of the CHP flue gas for test purposes. The process is operated to be water neutral. The recirculating water washes at the top of the absorber are used to control the depleted flue gas temperature/water vapor content. If water accumulates in the absorber-stripper loop, the flue gas temperature leaving the absorber is allowed to increase, increasing the water vapor content of the depleted flue gas, and vice versa.
2.2 Testing to support process characterization
The key performance indices are those features of the PCC process that are of interest when designing and planning for a full-scale implementation of the technology. Some of these indices can be modeled using chemical/thermodynamic/physical design data. A primary function of pilot-plant operations is to provide measured data such that uncertainties in the model can be reduced by comparison of model results with measured results.
The key performance indices are dependent parameters that can be expected to vary with changes in the process independent parameters. Performance data collected when changing the independent parameters during pilot-plant operations can be used to calibrate the process model, which can then be used to identify a set of independent parameters that “optimize” the key performance indices.
Pilot-plant operations can also be used to quantify those key performance indices that are not readily amenable to modeling including the effects of trace constituents of the flue gas supply. There are also intermittent and long-term performance indices that cannot be effectively modeled and must be assessed from many hours of pilot-plant operations (typically 1000s of hours) including: heat exchanger fouling, mass transfer packing fouling, foaming, material corrosion, solvent quality control measures, solvent loss/replacement, etc.
2.3 Pertinent independent parameters
The independent parameters are those temperatures, pressures, flows, compositions, and physical design parameters readily subject to control by the plant operators. Changing these parameters can be expected to affect the key performance indices (dependent parameters). The most important independent parameters for the purposes of modeling the process installed at TCM DA are listed below.
- Inlet flue gas characteristics
- CO2 content
- Flow rate
- Temperature
- With/without flue gas pre-treatment for SOX and particulates (future).
- Solution characteristics
- Amine concentration
- Circulation rate
- Lean solution CO2 loading.
- Equipment design characteristics
- Absorber height
- Lean solution flash/compression use
- Number of water washes
- Rich/lean heat exchanger effectiveness.
- Operating options
- Stripper pressure.
2.4 Modeled key performance indices (dependent parameters)
The set of key performance indices that can be modeled and quantified by pilot-plant operations at TCM DA are listed below.
- CO2 capture performance
- % CO2 captured / produced / emitted.
- Utility use
- Cooling duty
- Electrical power
- Steam thermal.
- Depleted flue gas amine/degradation product content.
2.5 Key performance indices not modeled (dependent parameters)
While it is fairly straightforward to model the heat and mass transfer associated with the PCC process, there are key performance indices that are less straightforward to model. It is more expedient to quantify these indices, which are listed below, by measurements during pilot-plant operations.
- Depleted flue gas trace constituents
- Mercury and air toxics
- Particulates
- SO2–SO3–NOX
- Total hydrocarbons (HC) – Amine/degradation products not modeled.
- Product CO2 trace constituents
- O2
- SO2–SO3–NOX
- Total HC–Amine/degradation products not modeled.
- Continuous waste streams
- DCC blowdown.
2.6 Long-term process/plant monitoring
There are also key performance indices that can only be assessed over many hours of operation. These include chronic effects as well as intermittent operations as shown below.
- Material uses
- Amine make-up
- Water make-up/blowdown.
- Intermittent waste streams
- Amine reclaim waste
- Lean-solution filter cake
- Spent activated carbon.
- Heat exchanger fouling/corrosion
- Gas-liquid contactor fouling/corrosion/foaming
- Accumulation/emission of degradation/corrosion products.
2.7 Key outcome
Key outcomes of pilot-plant operations are:
- A stand-alone model that predicts key performance indices within the uncertainty in actual measurements made during pilot-plant operations (or other clearly stated uncertainty) when only the independent parameters listed above are the variable inputs to the model
- One or more sets of formal performance test results collected during “base-case” operations that include, in addition to the modeled key performance indices, empirical measurement of the key performance indices not modeled. These “base-case” operations can be expected to be conducted under a set of independent parameters that have been determined to “optimize” the key pre-defined performance indices.
3. Performance testing principles
3.1 General performance testing guidelines
There is no accepted procedure for assessing PCC plant performance. There are, however, reference-testing procedures that are similar in scope and provide guidance for specifying the protocols under which the performance of PCC plants can be verified. These include:
- Overall power plant performance – Steam-boiler operations are comparable in complexity to PCC plant operations. Flow, temperature, and pressure, and composition data must be collected over the test period and are used to calculate a number of key performance indices such as steam temperature, pressure, and flow, fuel quality, flue gas flow rate and composition, sensible and latent heat losses in the flue gas, auxiliary power use, gross generation, net generation, etc. The overall power plant performance test code will also make extensive reference to companion test codes for measuring temperature, pressure, flow, gas composition, electrical and other power flows, and sub-component performance (boilers, air heaters, turbines, etc.). The American Society of Mechanical Engineers (ASME) publishes and maintains performance test codes for a wide range of equipment that have a long history of successful use [4].
- Quantifying flue gas emissions – The U.S. Environmental Protection Agency (EPA) has published reference methods for quantifying emissions from stacks for the purpose of demonstration conformance with the site air emission permit. These reference methods have a long history of use in the U.S. and have achieved wide acceptance. Appendix A lists the pertinent U.S. EPA reference methods. The European Commission has published similar reference methods.
The performance testing protocols presented here draw heavily on these two sources.
3.2 Base-case performance testing/process verification
Results from the base-case testing will be used to assess the steady-state performance of the process for the purposes of designing the full-scale plant and estimating capital and operating costs. For this reason, base-case performance testing should be conducted with measurement uncertainty as low as can be reasonably achieved. Therefore, test protocols consistent with well-developed reference methods should be incorporated as much as possible.
3.3 Parametric performance testing
The primary objective of parametric performance testing is to observe the effects on the key performance indices of incremental changes in the various independent variables. While accuracy in measurement is always desired, some bias error in measurements can be tolerated in parametric testing as long as the measurements achieve adequate precision; i.e., the measurement instruments give repeatable values. This condition can usually be met without strict adherence to reference methods that can be very costly to use as frequently as is required for a parametric performance testing program.
4. Test conduct and data collection procedures
4.1 Instruments and methods of measurement
4.1.1 Temperature
Process temperatures are generally not key performance parameters for a PCC plant. Nonetheless, temperature measurements are process condition indicators and care should be taken in their measurement.
No review of process temperature instrumentation was conducted in support of this study. In general, thermocouple or resistance temperature detectors are commonly deployed for process monitoring. These are usually precise enough to give acceptable repeatability without re-calibration. However, care should be exercised in ensuring that electrical temperature measurement signals are correctly wired, correct calibration algorithms are employed, and the resulting temperature is correctly logged and displayed to the operators.
4.1.2 Pressure
Process pressures are generally not key performance parameters for a PCC plant without a pipeline gas compressor. (Pipeline compressor discharge pressure would be a key performance parameter.) Nonetheless, several pressure measurements are process condition indicators and care should be made in their measurement. These include absolute and differential pressures at flow metering installations, absorber flue gas pressure drop, liquid distribution spray pressures, and stripper operating pressure.
No review of process pressure instrumentation was conducted in support of this study. In general pressure transmitters are commonly deployed for process monitoring. The key pressure transmitters, at a minimum, should be recalibrated according to manufacturer’s specifications prior to the onset of parametric testing. Pressure transmitters supporting primary flow measurement calculations should be recalibrated during base-case testing.
4.1.3 Flow
The standard used for flow metering is ASME PTC 19.5 Flow Measurement. Note that high accuracy may not be required for parametric testing where the incremental effect on key performance indices with incremental changes in process conditions is measured. In this case high precision (repeatability) may be an adequate substitute for high accuracy.
The flow meters installed in the PCC plant at TCM DA supporting CHP flue gas are listed in Table 2, respectively. The flow metering locations were indicated in Fig. 1. TORBAR pitot tube-style flue gas flow meters are the predominant choice implemented with single installation of an ultrasonic flow meter (after the DCC). Vortex flow meters are used to measure steam flows to the reboiler. A vortex flow meter is used to meter final CO2 product flow, which is redundant to the TORBAR flow meter.
The flow metering installations have been internally analyzed in detail at TCM DA, identifying the sources of uncertainty in each flow metering location.
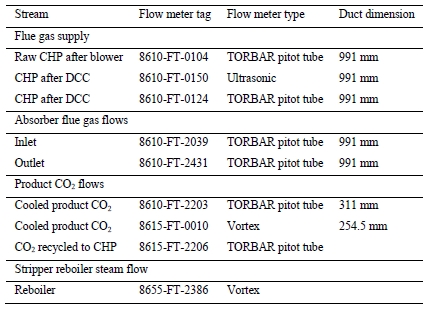
Table 2. Gas and steam flow metering for CHP flue gas applications at TCM DA.
4.1.3.1 TORBAR pitot tube flow meters
The uncertainty in the flow measurements using the TORBAR flow meters was estimated to be slightly greater than 2.5%. Of this, 2% was associated with installation of the TORBAR flow meters, by far the largest uncertainty component. This uncertainty component is a measure of the sensitivity of bias error introduced into the differential pressure indication by misalignment of the flow element in radial dimension and rotational orientation to the flow. The uncertainty associated with installation cannot be effectively estimated short of performing an in-situ flow calibration against a primary standard, and the assignment of 2% uncertainty to this component is somewhat arbitrary; misalignment could result in higher bias errors. Thus, while the flow reading calculated from the TORBAR measured pressure differential, absolute pressure, and temperature may have a precision of approximately 1.8% (precision excludes installation uncertainty), the uncertainty in accuracy may be significantly more than the estimate. The uncertainty associated with installing this class of flow meters generally disqualifies them for use in applications requiring predictable accuracy unless a relative accuracy test audit (RATA) has been performed for the field installation.
4.1.3.2 Vortex flow meter
A vortex meter is installed to meter product CO2. The vortex meter is redundant to a TORBAR meter located nearby. Vortex flow meters are shipped with a flow factor which, when multiplied by the vortex shedding frequency (an internal meter measurement) and fluid density, gives mass flow. The density must be derived from temperature, pressure, and composition measurements. These meters cannot be recalibrated short of performing an in-situ flow calibration against a primary standard.
A vortex flow meter is also used to meter steam flow to the reboiler. It is a linear device that indicates mass flow; thus the calibration range is based on mass flow. This meter is suitable for high accuracy mass flow measurements if it is calibrated under the following conditions:
- Steam flow over the full range expected during operations
- Calibration temperatures and pressures close to the operating temperature/pressure
- Calibration against standards traceable to the National Institute of Standards & Technology (NIST) or equivalent.
4.1.4 Composition
The standard recommended here for high-accuracy gas composition measurements is the use of reference standards commonly employed to monitor compliance with air emissions regulations. Where possible, the use of continuous emissions monitoring (CEM) methods is recommended.
It is recognized that the Fourier Transform Infrared (FTIR)-based systems installed at TCM DA will continue to be used. The relative locations for the sampling points are indicated in Fig. 1. The gas compositions reported by these instruments may be sufficiently accurate and precise to meet the requirements of the standards indicated, but this should be demonstrated against the instruments and procedures in the respective reference methods. The reference methods indicated below should be employed during all base-case testing unless there is clear evidence that the FTIR system gives results that duplicate the reference methods.
4.1.4.1 Flue gas supply and depleted flue gas
Table 3 lists the several flue gas components and the recommended reference methods for quantifying the components. CEMs are available for all non-condensable, non-soluble flue gas components. The condensable/soluble flue gas components and particulate matter require extractive sampling reference methods.
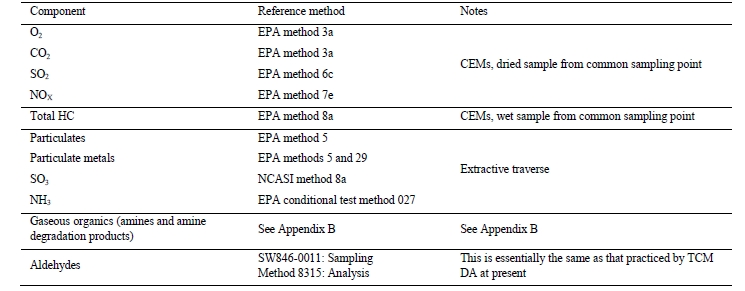
Table 3. Flue gas composition sampling and analysis reference methods.
4.1.4.2 Product CO2
Table 4 lists the several product CO2 components and recommended reference methods for quantifying the components. CEMs are available for all components except NH3.
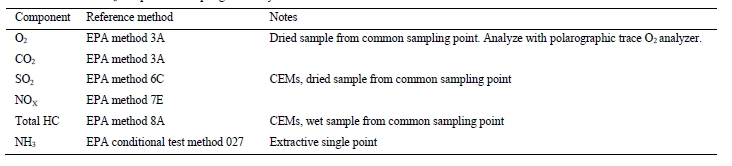
Table 4. Product CO2 composition sampling and analysis reference methods
The most critical parameters for delivery of the product CO2 to receiving pipelines are likely to be O2 content and moisture content. Measurement of trace O2 in any gas stream is challenging. In-situ O2 analyzers commonly used for measurement of flue-gas O2 at levels, which are typically above a few % (vol), are not sufficiently sensitive to accurately quantify trace levels of O2. Trace O2 levels may be quantified by polarographic (fuel cell) analyzers. Paramagnetic analyzers or gas chromatography may also be used but these are likely to add complexity and/or expense without significantly increasing accuracy. All of these techniques require extraction of a gas sample to the analyzer. Care must be exercised to exclude sampling system and instrument air in-leaks and to completely purge the sampling system of air on start-up and after calibrations; even small residues of air (containing 210,000 ppmv O2) will result in erroneously high analyses. Certified trace O2 calibration gases are also required. Moisture control will be part of a pipeline compression package that is not a part of the pilot plant at TCM DA.
Note that CO2 monitoring in the product CO2 stream is for reference only. Instrument readings near 100% cannot be relied on for accuracy at the 99.99% (vol) readings expected. Nitrogen is a likely diluent that can only be quantified by gas chromatography. An N2/O2 ratio cannot be assumed in the product CO2 equal to that in air. Dissolution of O2 in the aqueous amine solution or transfer of flue gas micro-bubbles with release in the stripper cannot be ruled out.
4.2 Instrumentation recommendations
4.2.1 Temperature measurements
- No pre-test calibrations required
- Loop checks should be made on temperature instruments supporting flue gas flow meters and product CO2 flow meters during parametric testing.
4.2.2 Pressure measurements
- Loop checks should be made on pressure instruments during parametric testing
- Pressure transmitters supporting flow meters and product CO2 flow meters should be recalibrated prior to or during all base-case test campaigns.
4.2.3 Flow measurements
- A RATA (see Appendix C) should be conducted, calibrating the three (3) flow metering installations for the CHP flue gas flow between the DCC and the absorber during each base-case test campaign. During this test, data may also be collected at the absorber outlet to calibrate the TORBAR flow metering installation at this location.
- Reboiler steam condensate orifice flow elements should be used to quantify reboiler steam use
- One of the following should be accomplished during base-case testing:
- A RATA (see Appendix C) to calibrate within 2% accuracy the TORBAR flow meter installed to meter the product CO2 flow
- A differential flow element consistent with ASME PTC 19.5 should be at an applicable location to achieve CO2 flow measurement within 2% uncertainty.
4.2.4 Composition measurements
- The FTIR analyzer system should be calibrated against primary calibration standards weekly or on a frequency that results in instrument drift of no more than 2% on calibration gases
- Gas stream sampling and analysis consistent with reference methods indicated in Table 3 and Table 4 should be employed during all base-case test campaigns
- Flue gas sampling ports should be used to sample from the duct near the existing flue gas flow meters
- The depleted flue gas sample should be taken from a probe extending at least 50 cm in from the absorber wall.
5. Calculation and reporting of key performance indices
Performance data collected during operations at TCM DA pilot plant fall generally into two broad classes: 1) data collected during parametric testing to support process model development and identify optimal operating conditions, and, 2) base-case data collected during operation under optimized conditions to verify the performance of the process, modeled parameters, and those key performance indices that are not modeled.
A complete test results report includes:
- List of independent parameters; those parameters under the more or less direct control of the operators that describe the process conditions imposed for the test
- Several key performance indices; dependent parameters that are uniquely determined by the process design and the independent parameters established by the operators.
5.1 Independent parameters
Table 5 lists the measured independent parameters that are likely to influence the key performance indices and should be included as test conditions in any report of process performance.
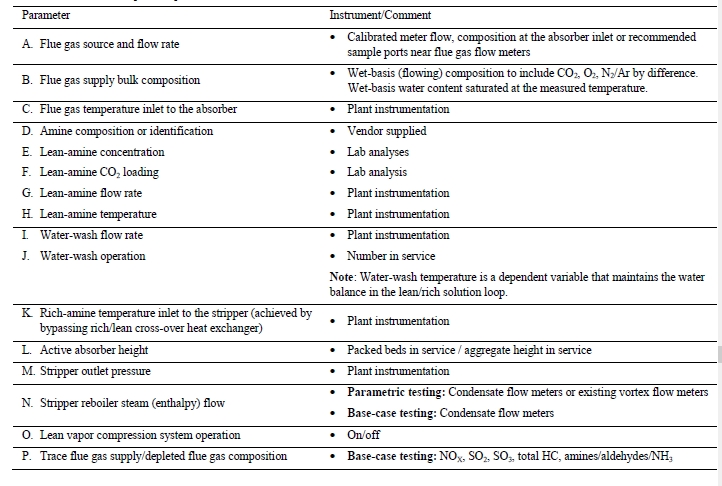
Table 5. Measured independent parameters.
Table 6 lists pertinent independent parameters derived from the measured independent parameters that are likely to be more instructive than the parameters from which they are calculated.
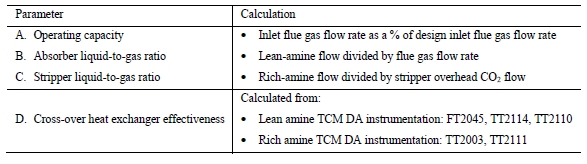
Table 6. Derived independent parameters.
5.2 Test period data results
Test period data include dependent variables that are directly measured parameters as well as key performance indices that are pertinent to calculations of measured values and independent parameters. Table 7 lists the important measured dependent parameters.
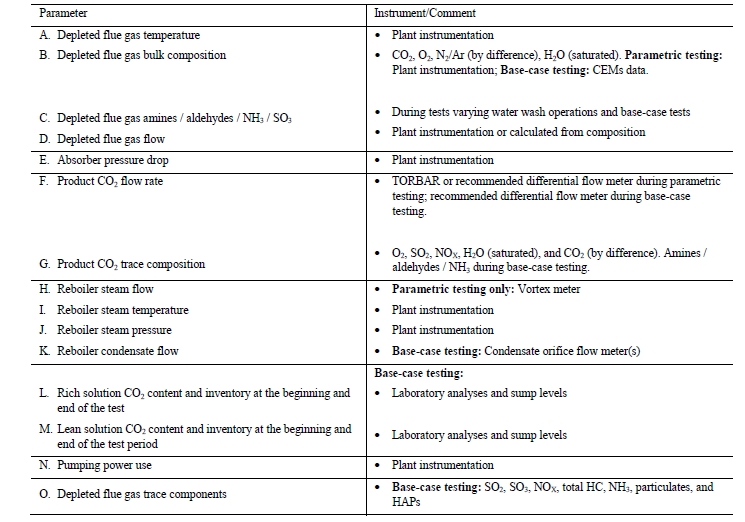
Table 7. Measured dependent parameters.
Table 8 lists the key performance indices. Each test period report should include these data.
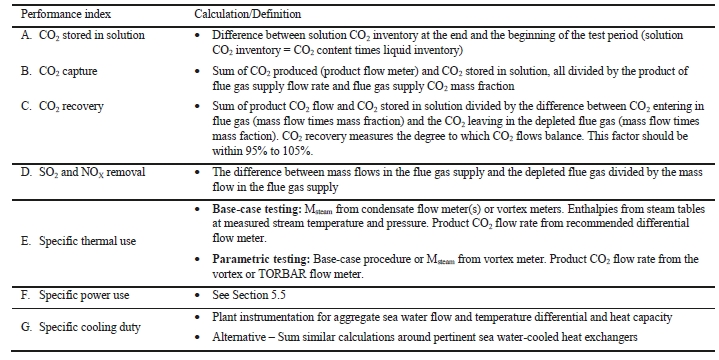
Table 8. Calculated key performance indices.
Fig. 3 lays out the general CO2 flows. Note that CO2 leakage to atmosphere is included as a flow. As leakage flows cannot be measured directly, it does not enter into the calculations. Its inclusion here is simply to acknowledge that leakage flow is a possibility. CO2 accumulation is the amount of CO2 stored within the amine pilot-plant boundaries over the course of a test; CO2 may accumulate in (or be released from) the rich/lean solution over the course of a test period.
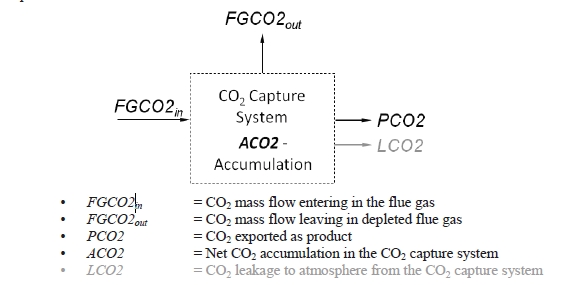
Fig. 3. CO2 capture flow diagram.
Three general methods of calculating CO2 capture efficiency are:
- The ratio of measured high-purity product CO2 flow to the CO2 entering the absorber in the flue gas is given by:
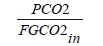
- The ratio of measured high-purity product CO2 flow to the sum of the high-purity product CO2 flow and the CO2 flow leaving the absorber in the depleted flue gas is given by:
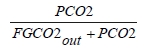
- The ratio of the difference between the CO2 entering the absorber in the flue gas and the CO2 leaving the absorber in the depleted flue gas to the CO2 entering the absorber in the flue gas is given by:
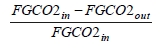
The relative uncertainties in CO2 capture by these three methods, using various combinations of flow meter data, were assessed. The conclusion is that uncertainty in CO2 capture is minimized in Method 2 above, assuming that the CO2 entering the capture plant is the sum of the two measured CO2 flows out of the plant: 1) PCO2 – High-Purity Product CO2 and 2) FGCO2out – CO2 Emitted in the Depleted Flue Gas Leaving the Absorber.
As the specific thermal use and specific cooling duty will be calculated using the measured product CO2 flow, the CO2 capture should also make use of the measured CO2 product flow. This recommends against Method 3, which uses only flue gas CO2 flows.
Key independent parameters that characterize CO2 capture plant performance include inlet flue gas flow rate as a % of design and absorber liquid/gas ratio, both of which use measured inlet flue gas flow rate. To the extent that absorber operation details are to be assessed and reported as key performance indices, corresponding reported CO2 capture should also be based on the measured inlet flue gas CO2 flow. This recommends against Method 2 despite its identification as the least uncertain method. In any event, sufficient data will be collected during operations to calculate and report CO2 capture by all methods.
Note that a 4th method might be considered using only dry-basis CO2 concentrations for the absorber inlet and depleted flue gas streams and assuming all dry components other than CO2 pass through the absorber unchanged. This 4th method requires no flow measurements and is given by:

For all test periods, CO2 recovery should be reported. This parameter is an indicator for the overall uncertainty in test results:
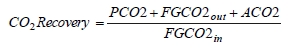
CO2 emissions are not included in the key performance indices listed in Table 8. Measuring CO2 emissions for the purposes of meeting air emissions regulations will likely require traverse sampling for composition and velocity from the stack.
CO2 emissions may be estimated by subtracting the sum of the (direct-measured) product CO2 flow (PCO2) and the CO2 stored in solution (ACO2, calculated) from the flue gas supply CO2 flow (FGCO2in). Note that this method of calculating CO2 emissions is a comparatively small difference in two large numbers and carries considerable uncertainty.
5.4 Specific thermal use
Specific thermal use is the heat supplied by imported steam, primarily to the stripper reboiler, divided by the product CO2 flow. The calculation for this parameter:
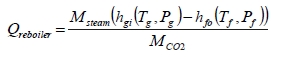
Details on each term in this equation are given in Table 9.
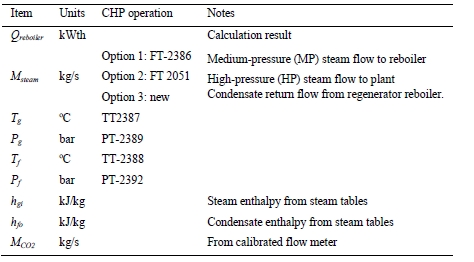
5.5 Electrical utility use
The primary auxiliary power uses for PCC are the induced draft (ID) fan (to overcome flue gas pressure drops in the plant), the aggregate of solution and water pumping inside the plant, and the CO2 compressor (to deliver at pipeline pressure; the TCM DA pilot plant does not have a CO2 pipeline compressor). The ID fan use will correlate most closely to flue gas flow rate. The internal pumping power loads will correlate loosely with CO2 production. Thus, it is unlikely that any single parameter will be useful in describing process auxiliary power use. In practice, pumping power differences from varying the independent parameters during parametric testing are likely to be insignificant. ID fan load will change with flue gas supply flow rate and, possibly, liquid flows in the absorber tower. Both of these factors are included in the ID fan pressure rise and flue gas flow rate. Auxiliary power use for a full-scale process can be estimated by:
- Summing the full-scale pumping loads
- Modeled ID fan power use from design flow rate and required pressure rise measured at pilot scale
- Modeled compressor power used to compress the product CO2 from stripper column overhead pressure and specified compressor discharge pressure to deliver to the receiving pipeline.
These can be developed from parameters included in Table 7 and a specified receiving pipeline pressure.
6. Conclusions
A generic CO2 capture testing methodology that has been applied at multiple sites providing details on the procedure, its key performance indices and their associated specifications, as well as the required pre-test work has been presented. Specific application of the methodology for the CO2 Technology Centre Mongstad site, a CO2 capture testing facility located in Norway that performed CO2 capture tests using MEA, is shown as an illustrative example.
Acknowledgements
The authors gratefully acknowledge the staff of TCM DA, Gassnova, Statoil, Shell, Sasol, and Aker Solutions for their contribution and work at the TCM DA facility.
The authors also gratefully acknowledge Gassnova, Statoil, Shell, and Sasol as the owners of TCM DA and Aker Solutions for their financial support and contributions.
Appendix
References
- Hamborg E S, Smith V, Cents T, Brigman N, Falk-Pedersen O, De Cazenove T, Chhaganlal M, Feste J K, Ullestad Ø, Ulvatn H, Gorset O, Askestad I, Gram L K, Fostås B F, Shah M I, Maxson A, Thimsen D. Results from MEA testing at the CO2 Technology Centre Mongstad. Part II: Verification of baseline results. Energy Procedia; 2014.
- Brigman N, Shah M I, Falk-Pedersen O, Cents T, Smith V, De Cazenove T, Morken A K, Hvidsten O A, Chhaganlal M, Feste J K, Lombardo G, Bade O M, Knudsen J, Subramoney S C, Fostås B F, De Koeijer G, Hamborg E S. Results of amine plant operations from 30 wt% and 40 wt% aqueous MEA testing at the CO2 Technology Centre Mongstad. Energy Procedia; 2014.
- Morken A K, Nenseter B, Pedersen S, Chhaganlal M, Feste J K, Tyborgnes R B, Ullestad Ø, Ulvatn H, Zhu L, Mikoviny T, Wisthaler A, Cents T, Bade O M, Knudsen J, De Koeijer G, Falk-Pedersen O, Hamborg E S. Emission results of amine plant operations from MEA testing at the CO2 Technology Centre Mongstad. Energy Procedia; 2014.
- Performance test code on overall plant performance. ASME PTC-46. ASME. New York, NY; 1996.
- Certification of calibration traceable to the NIST. Colorado Engineering Experiment Station, Inc. 10EHA-0001_1: 24; August 2010.
- Working group review of sampling and analytical methods for amines and amine degradation products in post-combustion carbon capture technologies. EPRI. Palo Alto, CA; 2012; 1026867.
- PTC 19.5 flow measurement. ASME. New York, NY; 2004.
- Guidelines for flue gas flow rate monitoring. EPRI. Palo Alto, CA: 1995; TR-104527.
- Fluid flow in closed conduits using tracers: Measurement of gas flow by transit time using radioactive tracers. BS 5857-2.4; 1980.
Results from MEA testing at the CO2 Technology Centre Mongstad. Part II: Verification of baseline results (2014)
Espen S. Hamborga,b, Vian Smitha,c, Toine Centsa,c, Natasha Brigmana,c, Olav Falk- Pedersena,d, Thomas De Cazenovea, Milan Chhaganlala,b, Jane K. Festea,b, Øyvind Ullestada,b, Helge Ulvatna,b, Oddvar Gorsete, Inga Askestade, Lars K. Gramf, Berit F. Foståsb, Muhammad I. Shahd, Andrew Maxsong, David Thimseng,*
aCO2 Technology Centre Mongstad (TCM DA), 5954 Mongstad, Norway bStatoil ASA, P.O. Box 8500, 4035 Stavanger, Norway cSasol Technology, P.O. Box 5486, Johannesburg 2000, South Africa dGassnova SF, Dokkvegen 10, 3920 Porsgrunn, Norway eAker Solutions, P. O. Box 222, 1326 Lysaker, Norway fFORCE Technology, Park Allé 345, 2605 Brøndby, Denmark gElectric Power Research Institute, 3420 Hillview Avenue, Palo Alto, CA 34304, USA *Corresponding author

Abstract

Independent verification protocol (IVP) work has been conducted at the CO2 Technology Centre Mongstad (TCM DA) during treatment of flue gas from a natural gas-fired combined heat and power (CHP) plant. The testing applied an aqueous 30 wt% monoethanolamine (MEA) solvent system treating flue gases with a flow rate of about 47.000 Sm3/hr and a CO2 content of about 3.5%. The CO2 capture rate was about 90% and the thermal steam consumption was about 4.1 GJ/t CO2. Emissions of MEA were very low and MEA-related degradation products were all below detection levels, and all within the emission limits set by the Norwegian environmental authorities. The current work may be considered an independently verified baseline for a non- proprietary post-combustion amine based solvent system carried out at an industrial-scale plant facility.
Long-term performance indices, such as material corrosion, MEA solvent degradation, etc., have not been considered in the current IVP work. Additional minor process adaption to the aqueous MEA solvent system, such as increased MEA concentrations, the use of anti-foam solutions, etc., may lead to lower thermal steam consumptions than aforementioned.
1. Introduction
CO2 Technology Centre Mongstad (TCM DA), located next to the Statoil refinery near Mongstad, Norway, is one of the largest post-combustion capture (PCC) test facilities in the world. TCM DA is a joint venture between Gassnova, Statoil, Shell, and Sasol. The purpose of this facility, which started operation in August 2012, is to allow vendors of suitable amine formulations and other PCC processes to test their technology and collect performance data to support full-scale design and anticipate the associated performance and operating costs. A unique aspect of the facility is that either a slipstream from a natural gas-fired combined heat and power (CHP) plant or an equivalent volumetric flow from a refinery residue fluid catalytic cracker (RFCC), whose higher CO2 content (about 12.9% compared with about 3.5% for the natural gas-based flue gas) is closer to that seen in coal flue gas, can be used for CO2 capture. In the CHP plant, the natural gas is combusted in a gas turbine and the flue gas content and characteristics are similar to those of a combined cycle gas turbine (CCGT) power plant. One of the testing facilities in place at TCM DA is a highly flexible and well-instrumented generic amine plant, designed and constructed by Aker Solutions and Kværner, aimed to accommodate a variety of technologies with capabilities of treating flue gas streams of up to 60,000 Sm3/hr. This plant is being offered to vendors of solvent-based CO2 capture technologies to primarily test: (1) the performance of their solvent technology; and (2) technologies aimed to reduce the atmospheric emissions of amines and amine-based degradation products from such solvent-based CO2 capture processes.
An independent verification protocol (IVP) has been developed by the Electric Power Research Institute (EPRI)
to be used as part of the overall performance assessment of amine-based TSA processes, as described in details elsewhere [1]. The IVP is designed to provide a structured testing procedure for assessing thermal and environmental performance of PCC processes under normal operating conditions.
The IVP has been applied during base-case testing done 6–10 January 2014 on the TCM amine plant using aqueous 30 wt% monoethanolamine (MEA) as the solvent while treating flue gas at a flow rate of about 47.000 Sm3/hr from the CHP plant. The IVP project was performed jointly between TCM DA, Aker Solutions, FORCE Technology, and the Electric Power Research Institute (EPRI), and the base-case testing is part of Aker Solutions’ test campaigns at TCM DA.
This work is part of a continuous effort of gaining better understanding of the performance potential of the non- proprietary aqueous MEA solvent system, conducted by TCM DA and its affiliates and owners, in order to test, verify, and demonstrate CO2 capture technologies [1, 2, 3]. The purpose of the current work is to provide the results of the IVP done for aqueous 30 wt% MEA, which provides a baseline that can be commensurately compared against other (solvent-based) PCC processes. This work may thus be considered the baseline for a non-proprietary PCC amine-based solvent system treating low CO2 partial pressure flue gases at a significant flow rate from the combustion of natural gas in a gas turbine.
2. Project overview
The TCM pilot-scale amine plant was designed and constructed by Aker Solutions and Kværner. The amine plant was designed to be flexible to allow testing of different configurations, and has respective capacities of about 80 and 275 tonnes-CO2/day for CHP and RFCC flue gas operations. The TCM DA amine plant process flow diagram showing high-level equipment contained within the plant along with key extant instrumentation and the nominal CHP flue gas characteristics is given elsewhere [1]. The major systems include:
- An induced draft (ID) blower to overcome pressure drops and blow the flue gas through the plant with a blower output capacity of up to about 270 mbar and 70,000 Sm3/hr.
- A direct-contact cooler (DCC) system to initially quench and lower the temperature and saturate the incoming flue gas by a counter-current flow water in order to improve the efficiency of the absorption process and provide pre-scrubbing on the flue gas. The DCC system has two individually operated packed columns for operations with respectively the CHP flue gas and the flue gas from the refinery cracking unit. The DCC column designed for CHP flue gas operations has of a 3-m diameter and a total of 16 m of height. The section where water counter currently contacts the flue gas is of 3.1 m of height with Flexipack 3X structured stainless-steel packing of Koch Glitsch. The DCC column designed for the flue gas from the refinery cracking unit has a diameter of 2.7 m and a total height of 16 m. The section where water counter currently contacts the flue gas is of 3 m of height with Intalox Snowflake random polypropylene packing of Koch Glitsch.
- An absorber to remove CO2 from the flue gas using solvent. The absorber has rectangular polypropylene-lined concrete column with a cross-section measuring 3.55 x 2 m of a total of 62 m of height. The lower regions of the tower, where the amine solution contacts the flue gas, consist of three sections of Koch Glitsch Flexipac 2X structured stainless-steel packing of 12 m, 6 m, and 6 m of height, respectively. Water-wash systems are located in the upper region of the tower to scrub and clean the flue gas particularly of any solvent carry over, and consist of two sections of Koch Glitsch Flexipac 2Y HC structured stainless-steel packing of both 3 m of height. The water wash system is also used to maintain the water balance of the solvent system by adjusting the temperature of the circulating water of the upper water-wash section. Liquid (re-)distributors, liquid collector trays, and mesh mist eliminators by Koch Glitsch are located at various locations in the tower. The CO2 depleted flue gas exits the absorber column to the atmosphere through a stack located at the top of the absorber column.
- Stripper columns to recover the captured CO2 and return CO2-lean solvent to the absorber. The amine plant consist of two independent stripper columns with overhead condenser systems; one measuring 1.3 m in diameter and a total of 30 m of height, the second measuring 2.2 m in diameter and also a total of 30 m of height. The lower regions of both stripper column, where the amine solutions is stripped, consist of Koch Glitsch Flexipac 2X structured stainless-steel packing of 8 m of height, and in the upper regions of the strippers consist of a rectifying water-wash section of Koch Glitsch Flexipac 2Y HC structured stainless-steel packing of 1.6 m of height. Liquid (re-)distributors, liquid collector trays, and mesh mist eliminators by Koch Glitsch are located at various locations in the strippers. Each stripper column is connected to its respective stream-driven thermosiphon reboiler system, providing the necessary heat required for the stripping process. The two stripper columns are operated independently considering the CO2 content in the flue gas, due to column design and hydraulics and gas velocities effects, i.e., the smaller diameter stripper column is utilized when treating CHP flue gas, whereas the large diameter column is utilized when treating flue gases of higher CO2 content.
- A set of pumps used to move the CO2-lean and CO2-rich solvent streams between the absorber and stripper and through a cross-flow heat exchanger to recover heat from the lean stream.
- A reflux drum, condenser, and pumps to dry the product CO2 that exits from the stripper. A portion of the product CO2 can also be recycled back to the inlet of the DCC to increase the concentration of the CO2 in the inlet flue gas stream.
The roles and responsibilities of the organizations that conducted the current IVP project are as follows:
- TCM DA is the prime on the project and its personnel organized the field testing including contracting to do gas sampling during the test period. Personnel from TCM DA and TCM DA owner organizations were responsible for planning and setting the test program for the base-case testing, and also operating the plant throughout. TCM DA personnel collected samples during the base-case testing for quantification of trace species in the depleted flue gas stream.
- Aker Solutions is the technology vendor testing its solvent-based PCC technologies at TCM DA. A part of Aker Solutions’ test period was to conduct a campaign based on the non-proprietary MEA solvent system, which was intended to be used as a reference for future testing. The base-case testing done 6–10 January 2014 was consequently a part of Aker Solutions’ test campaigns at the TCM DA amine plant.
- FORCE Technology brought a single sampling crew on-site during the base-case testing to extract and analyse samples from the CHP flue gas supply, depleted flue gas, and product CO2 streams. This sampling was conducted sequentially with a single set of continuous emissions monitors (CEMs). FORCE Technology also collected gas samples for off-site analysis of particulate, SO2/SO3, and amine-related compounds.
- EPRI was contracted to develop the IVP and help apply it during the base-case MEA testing. Two EPRI engineers were on-site during the testing to observe the conduct of the tests. EPRI is also the lead on the current IVC work.
3. Independent verification protocol approach
Base-case testing of the performance of the TCM amine plant using a nominal 30% MEA as the solvent was conducted the week of 6 January 2014 after approximately 6 weeks of operating the amine plant with the 30% MEA solution. The plant was operated at steady state through the entire week. (Note: The MEA solution concentration did drift down approximately 1 percentage point during the week of base-case testing.) The only operational abnormality was a short loss of flue gas flow for about 15 minutes at 15:00 hrs on 8 January 2014 from which operations were quickly restarted.
FORCE Technology was on-site to manually collect samples sequentially from the flue gas supply, depleted fuel gas, and product CO2. During all sampling periods the following sample data were collected:
- CO, CO2, NOX, O2, SO2, and N2 (by difference) concentrations in vol%
- Flow rate, pressure, and temperature.
The sampling time periods and sampling period designator are shown in Table 1 along with additional sampling undertaken on each day. Data logs for all sampling periods containing pertinent flows, temperatures, pressures, and concentrations measured by permanent plant instruments were supplied by TCM DA.
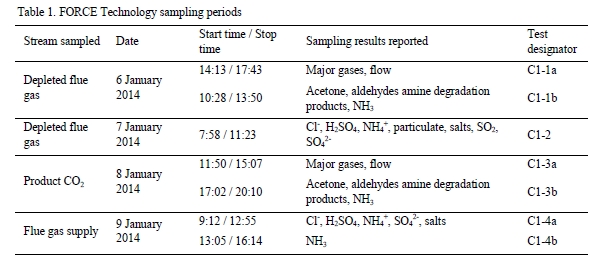
Table 1. FORCE Technology sampling periods.
4. Instrument assessment
This section assesses the quality of the instrumentation installed for measuring the respective compositions and flow rates. There are two measures of instrumentation quality:
- Accuracy / bias – Measure of the difference between the instrument reading (or average of a set of readings under unchanging process conditions) and the true value of the parameter. The “true value” must be determined by means other than the measurement in question. This is usually accomplished by simultaneous measurement of the parameter by the plant instrument and a reference method or instrument with calibration that can be traced to primary standards.
- Precision – Variability of the instrument reading when stream conditions do not change. Precision is a measure of the random error associated with the measurement.
The aggregate uncertainty in a measurement includes both precision error and bias error. Absent a calibration against primary standards, the uncertainty published by the instrument supplier is only the precision error.
Note also that precision is a measure of repeatability when the process parameter being measured does not change. It is often the case that the process parameter (flow, pressure, and temperature) does change over the measurement period. Thus, measurements over long periods of time (greater than process time constants) will also include an error term related to process uncertainty.
4.1 Gas phase compositions
The CO2 and O2 content of the flue gas supply, depleted flue gas, and CO2 product stream is routinely determined by the respective plant Fourier Transform Infrared (FTIR) (Applied Instrument Technologies and Finetech, model: Anafin 2000) and O2 (Siemens, model: Oxymat 6) sampling and analysis system. The sampling system admits the gas stream, sampled from various single points as given by Thimsen et al [1]. The sample is continuously drawn by a selection system serving the analyzer. The gas supply samples are diverted to the common analyzers in a 90-minute cycle, i.e., the analyzer cycles between flue gas supply for 15 minutes, depleted flue gas for 30 minutes, and CO2 product stream for 15 minutes. In each sampling, the analyzer sampling lines and cells are sufficient flushed with the gas to be measured and, after a certain time, wet-gas concentration for every 1½ minutes for a total of 10 concentrations are reported. The plant control system displays to the operators the most recent concentration report. Thus, the last report of the 10 is displayed for approximately 75 minutes until the next sampling cycle for the flue gas supply and CO2 product stream and approximately 60 minutes for the depleted flue gas.
The flue gas supply, depleted flue gas, and CO2 product stream compositions were analyzed by FORCE Technology during the base-case operations. The measurements reported by FORCE Technology were on a dry basis. (The sample is dried before analysis.) These dry-basis data were converted to wet basis by assuming that the flue gas supply is saturated with water at the temperature and pressure measured by the plant data acquisition system. The recalculated FORCE Technology data are given in Fig. 1 and Fig. 2, and compared to the values determined by the FTIR system. Details include:
- Fig. 1 displays the CHP flue gas supply CO2 and O2 concentration data over the test campaign. The agreement between FORCE Technology O2 measurements and those measured by TCM DA O2 analyzer on 9 January are as good as the agreement in respective CO2 measurements. These data show that for the last 2½ days of the campaign, CHP flue gas supplied to the pilot plant was of relatively uniform composition. This is probably not the case for the first 1½ days of the campaign. The variability in CO2 and O2 concentrations are significantly greater than the precision uncertainty in the measurements indicating that the changes in measured concentration represent real changes in CHP flue gas composition.
- Fig. 2 displays the depleted flue gas CO2 and O2 concentration data over the test campaign. The FORCE Technology O2 data collected on 6 January differ significantly from the TCM DA O2 data for the first half of the sampling period, but are in general agreement over the last half of the sampling period. The relative uniformity of the FORCE Technology data on 6 January suggests that the TCM DA O2 data above 15% O2 may be spurious and not a result of process changes. There was significant variation in the depleted flue gas FORCE Technology CO2 and TCM DA FTIR concentration data for the sampling period. The precision error for this measurement is in excess of 20%. In addition, there was a significant positive bias in the FTIR data compared to FORCE Technology data taken simultaneously on 6 January. The bias could be corrected by multiplying the FTIR data by 0.7 over this time period. Although the bias is significant, the error was about 0.1% points.
- The product CO2 composition data reported by FORCE Technology include O2 content between 1–2%. It is difficult to imagine a mechanism by which the product CO2 stream (stripper overhead) can contain this much oxygen, and it is therefore presumed that this oxygen is due to air in-leakage into the sampling system, thereby disqualifying the data. For the purposes of calculating CO2 removal and recovery, it is assumed here that the product CO2 stream consists only of CO2 saturated with water at the measured temperature and pressure.
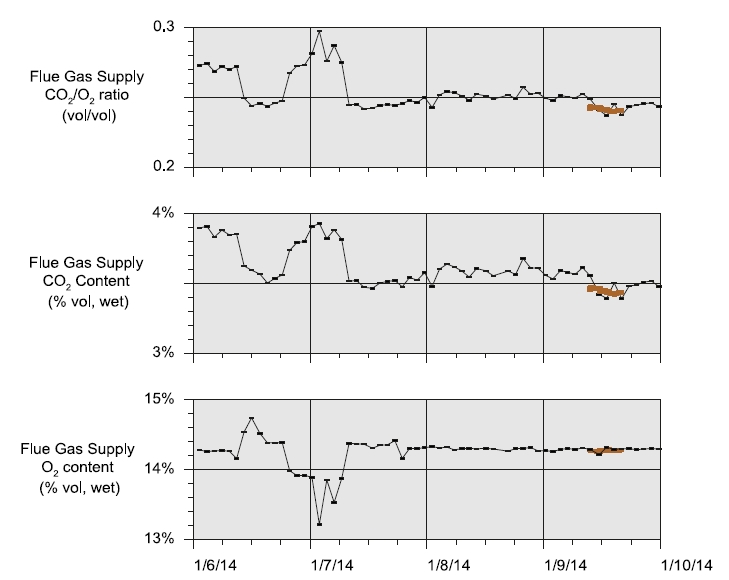
Fig. 1. CHP flue gas supply CO2 and O2 data. FTIR and O2 analyzer data are averaged over analysis circles. Data collected by FORCE
Technology on 9 January are also shown.
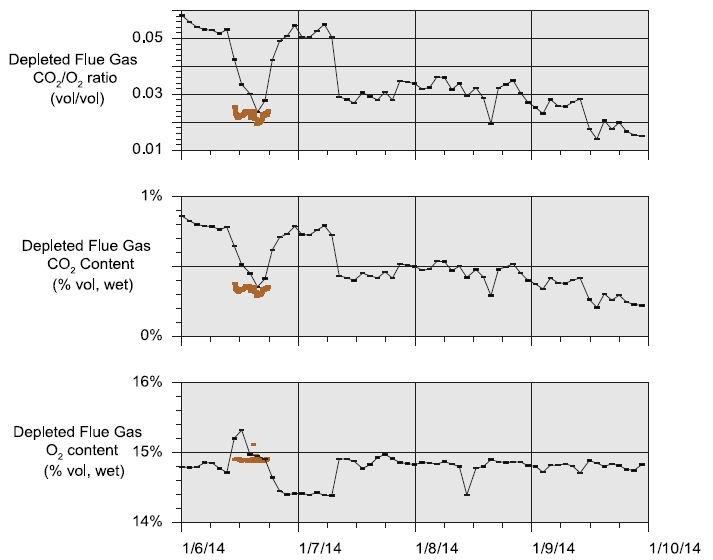
Fig. 2. Depleted flue gas CO2 and O2 data. FTIR and O2 analyzer data are averaged over analysis cycles. Data collected by FORCE Technology
on 6 January are also shown.
4.2 Gas phase flow rates
The flow rates of the flue gas, depleted flue gas, and CO2 product stream are continuously determined by plant instrumentation. The TCM DA amine plant facility is particularly well instrumented for determining the flue gas supply flow rate, with several different types of flow meters in series.
The flue gas, depleted flue gas, and CO2 product stream flow rates were determined by pitot-tube traversing during the base-case operations by FORCE Technology and the results compared to plant instrumentation are discussed below:
- The CHP flue gas supply flow is measured by two instruments, an ultra-sonic flow meter (FT-0150) and a multi- pitot-tube flow meter (FIC-0124), which are characterized in Table 2. The data from these flow meters are shown in Fig. 3. The flow rates are defined standard conditions of 15 °C and 1 atmosphere. The CHP flue gas flow was very steady over the test week with the exception of a 15-minute period on 8 January when the flow went to zero due to a trip of the ID blower. FORCE Technology made an independent measurement of flow on 9 January as indicated in Fig. 3. The difference between the value measured by FORCE Technology and that measured by the plant instruments is less than 1%. This result must be tempered by the reported uncertainty in the FORCE Technology measurement of 10%. The test period flow averages used for all calculations are the data reported by the ultrasonic flow meter (FT-0150).
- The depleted flue gas flow is measured by a single multi-pitot tube flow meter whose characteristics are listed in Table 2. The depleted flue gas flow rate of this instrument varies in a fashion that is uncorrelated with any known operational parameter rendering this data of little use for the purposes of the base-case testing. Investigation of this has indicated variation of the measured flue gas flow rate with the ambient air pressure. This may be related to the physical installation position of the instrument; however, exact cause for this flow rate variation is not yet understood. FORCE Technology measured a flow of 47.000 Sm3/hr (±10%) at this location on 7 January 2014.
- The key product CO2 flow meters are listed in Table 2. The product CO2 flow measured by the vortex flow meter (FT-0100) is the primary flow meter used by TCM operators. The data from this flow meter are shown in Fig. 4. The product CO2 flow was relatively steady over the test week with the exception of the 15-minute period on 8 January 2014 when the flow went to zero due to an ID blower trip. FORCE Technology made an independent measurement of flow on 8 January as indicated in Fig. 4. The difference between the value measured by FORCE Technology and that measured by the plant instruments is approximately 6%, within the uncertainty reported by FORCE Technology measurement of 10%.

Table 2. Key flow instrumentation. Precision uncertainties are based internal instrument assessment by TCM DA.
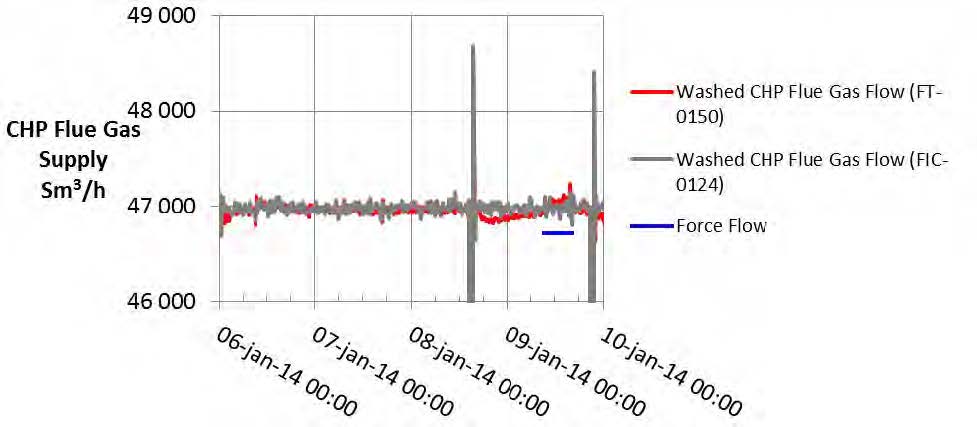
Fig. 3. CHP flue gas supply flow measurements.
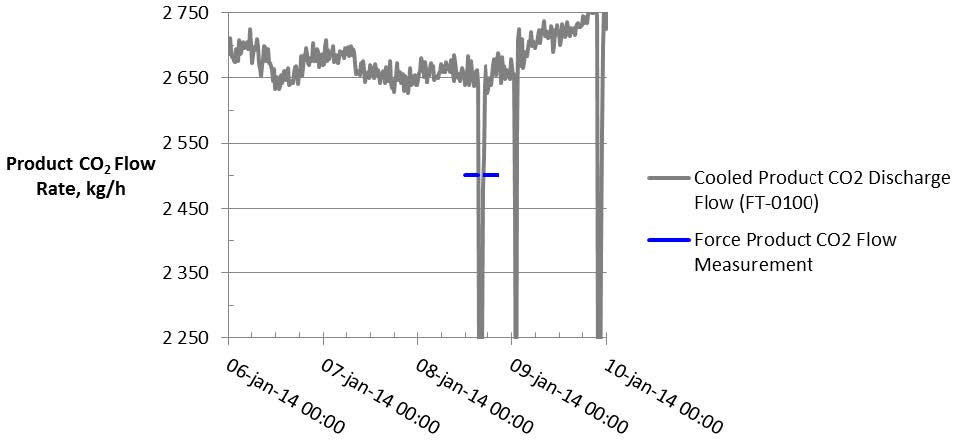
Fig. 4. Product flue gas flow rate and test period averages.
4.3 Steam and condensate flow rates
A schematic of the system supplying steam to the stripper reboiler is shown in Fig. 5. High-pressure (HP) steam is delivered from the refinery to the TCM amine plant at a pressure of approximately 30 bars, superheated to approximately 240°C to 310°C. The HP steam is throttled to a pressure near the stripper reboiler steam pressure at approximately 5 bars and then desuperheated with condensate. The stripper reboiler condensate collects in a receiver from which it is returned to the refinery. A small amount of medium-pressure (MP) steam is reduced to a lower pressure for use in steam heat tracing. The low-pressure (LP) steam condensate is returned to the same receiver as the stripper reboiler condensate.
The parameter of interest is the steam flow to the reboiler. A check on this parameter is the HP condensate flow returned to the refinery. The condensate return flow should be the sum of the reboiler steam flow and any condensate flow produced in steam heat tracing. Fig. 5 shows these two parameters. The condensate return flow indicated (FT- 2455) is consistently higher than the reboiler steam flow (FT-2386) by typically 2% to 8%. This difference is in the correct direction when heat tracing condensate (not measured by the reboiler steam flow meter) is entering the condensate receiver.
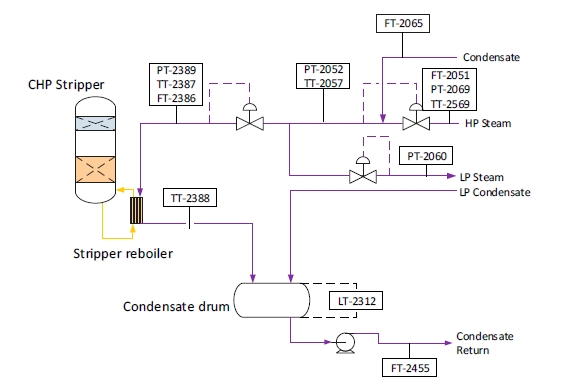
Fig. 5. Stripper reboiler steam supply flow schematic.
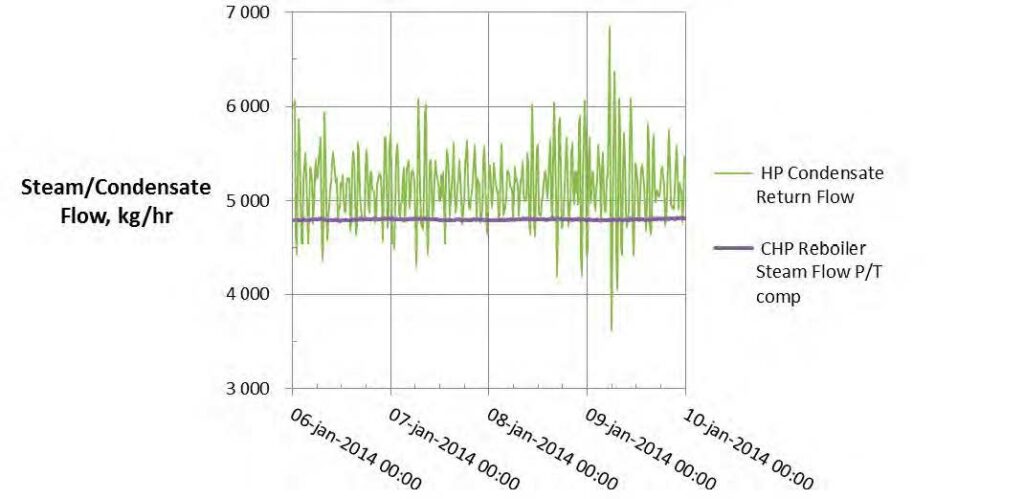
Fig. 6. Reboiler steam flow and HP condensate return flow.
5. Results and discussions
5.1 CO2 capture efficiency and recovery
CO2 capture efficiency can be quantified in four ways as described by Thimsen et al. [1] and indicated in Table 3. In addition, the CO2 recovery calculation is given in Table 3. The CO2 recovery is a measure of the CO2 mass balance.
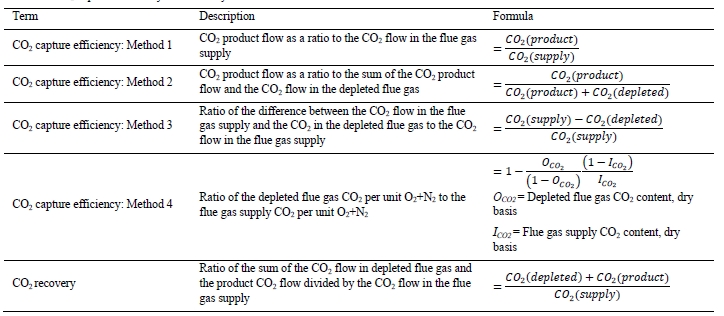
Table 3. CO2 capture efficiency and recovery calculations.
The depleted flue gas flow measurement is not yet a reliable measurement. A value can be calculated for the depleted flue gas flow by assuming that the oxygen and nitrogen entering the absorber with the flue gas supply leaves in the depleted flue gas. The depleted flue gas temperature may be used to calculate saturated water content. The depleted flue gas CO2 concentration may be used to calculate CO2 flow. Note that these are essentially the same assumptions as those used for Method 4, hence the Method 3 and Method 4 calculations result in essentially identical CO2 capture rates. Using the calculated flow of depleted flue gas allows an estimate of the CO2 recovery to be calculated.
Table 4 shows the four calculations of CO2 capture and recovery for the base-case test periods (using the calculated value for depleted flue gas flow). The first thing to note is that all calculated CO2 captures were fairly steady for the first three days of operation (test periods C1-1a to C1-3b). The CO2 capture on the last day (C1-4a, C1-4b) was significantly higher by approximately 3–4 percentage points. The CO2 recovery (mass balance) was neither greater than 95.5% nor as low as 91.3%. Note also that the CO2 capture calculated by Method 1 is always less than the CO2 capture calculated by Methods 2, 3, and 4. These two facts suggest that either quantification of CO2 flow in the CHP flue gas supply is biased high or that calculation of CO2 flow in the product is biased low.
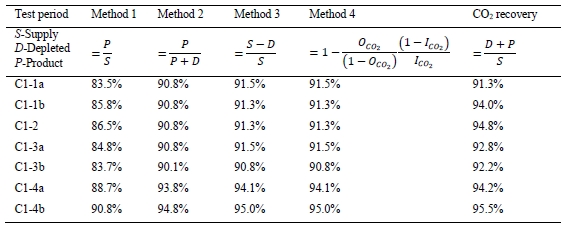
The uncertainty in measurement of flow and composition propagate into uncertainty in the CO2 capture. The uncertainty calculations and representative results from the each of the calculation methods are shown in Table 5. The following assumptions are used:
- Flow metering uncertainties are those theoretically estimated and calculated by internal work at TCM DA for the indicated flow meters [1]
- Concentration uncertainties for the flue gas flows are those aforementioned
- Concentration uncertainty for the product CO2 is arbitrarily assigned to be 2%, which allows for actual CO2 content as low as 98%
- CO2 capture percentage of 90% is representative of that measured during base-case testing. (The calculation is not particularly sensitive to this parameter between 85 and 95%.)
A few notes on the CO2 capture uncertainty results:
- The uncertainty in CO2 capture is almost all due to uncertainty in CO2 content of the CHP flue gas supply for the assigned total flow uncertainties. The CO2 capture uncertainty is relatively insensitive to both the product CO2 content uncertainty and the depleted flue gas CO2 content uncertainty.
- The fact that CO2 recovery is less than 100% suggests that one or more of the flows has a significant bias error than calculated from instrument specifications. Hence the need for a relative accuracy test audit of the pertinent flow meters to assign more realistic uncertainties. These are likely to be higher than the calculated values, which will increase overall CO2 capture uncertainty above that indicated in Table 5.
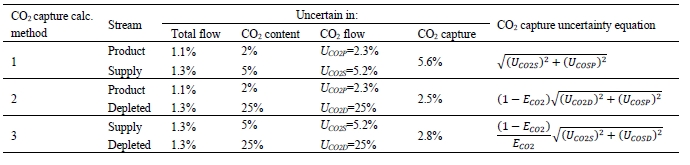
Table 5. Uncertainty in CO2 capture as a function of flow/composition measurement uncertainty (Nominal CO2 capture of ECO2 = 90%).
The heat released in the reboiler is calculated as the difference between steam enthalpy at the measured reboiler inlet temperature (T) and pressure (P) and saturated water enthalpy at the reboiler condensate temperature. The pertinent data are given in Table 6.
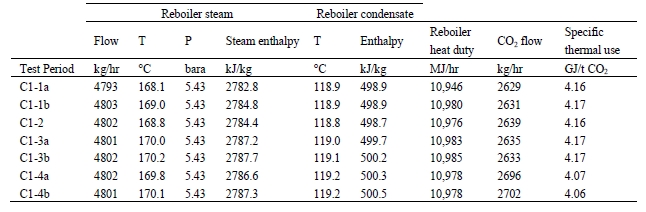
Table 6. Stripper reboiler thermal use calculation
The thermal steam consumption data give in Table 6 are based on aqueous 30 wt% MEA solvent system without the addition of any anti-foam solution. Upon addition of anti-foam solution and increase of the MEA solvent concentration during the MEA test campaign at TCM DA, the steam consumption was further reduced during CHP flue gas treatment, as described by Brigman et al [2]. Those tests were not a part of the current IVP work. Additionally, TCM DA has a LVC system installed; however, this system was not operated during Base-Case test and is consequently also not a part of the current IVP work. LVC systems have previously been showed by Knudsen et al. [4] to substantially decrease the thermal steam consumptions during amine plant operations with the aqueous MEA solvent systems.
5.3 Process contaminants
FORCE Technology measured gas-phase concentrations of the compounds listed below and the results are provided in Table 7. During the base-case testing time period, the CHP plant received refinery gas from the Mongstad refinery, which was, to some extent, co-fired with the natural gas.
- SO2 concentrations were measured on different days. The CHP flue gas supply SO2 concentrations are very low as are concentrations in the other streams.
- H2SO4 concentrations were measured in the two flue gas streams on different days. The flue gas H2SO4 concentrations are very low as are concentrations in the other streams. The H2SO4 concentrations were determined by extracting aqueous H2SO4 containing droplets, referred to as SO3 mist droplets, on a heated filter.
- NOx concentrations were below detectable limits for all streams
- Total particulates concentrations were measured on different days. The CHP flue gas supply total particulate concentrations are very low and were below detection limit in the depleted flue gas.
- Acetone, acetaldehyde, and formaldehyde were measured in the depleted flue gas and the product CO2 stream on separate days. The emissions concentrations of acetone and the aldehydes are higher in the product CO2 than the depleted flue gas, likely due to the low temperature boiling point nature of these compounds.
- NH3 concentrations were measured for both depleted flue gas and product CO2. The results indicated emissions of NH3, likely arising from MEA degradation process occurring in the solvent system.
- MEA concentrations were determined by iso-kinetic sampling conducted by TCM DA personnel and further sample analysis by the TCM DA laboratories. The MEA concentrations in the depleted flue gas are very low, and were below the emission limits set by the Norwegian environmental authorities (Miljødirektoratet). [3]
- MEA degradation products were determined by iso-kinetic sampling from the depleted flue gas and product CO2 by FORCE Technology and further laboratory analysis. The concentrations of any nitrosamines and nitramines were all below detection limits for both the depleted flue gas and the CO2 product. [3] The emissions of MEA degradation products were below the emission limits set by the Norwegian environmental authorities.
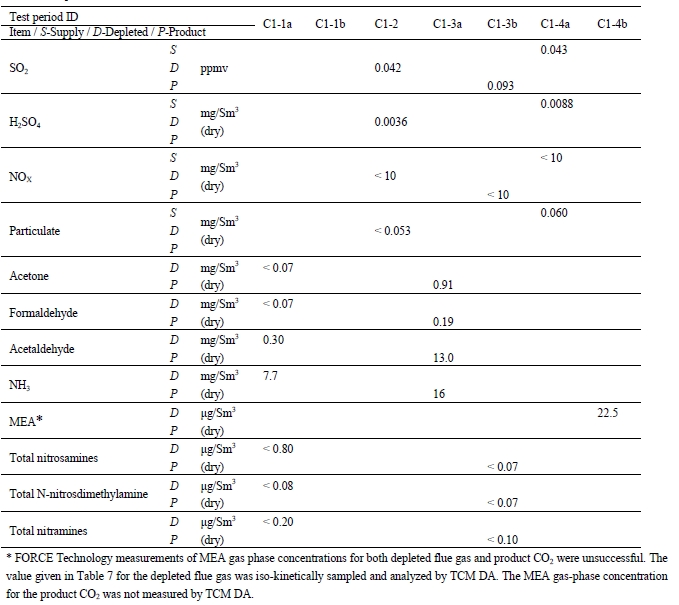
Table 7. Gas-phase concentrations.
5.4 Process stream information
Additional amine plant process information for the base-case test is given in Appendix A. This information is not covered by the current IVP work, but is given for the convenience of the reader.
6. Conclusions
IVP work has been conducted at CO2 Technology Centre Mongstad during treatment of flue gas from a natural gas-fired combined heat and power (CHP) plant. The testing is referred to as the base-case testing, applying an aqueous 30 wt% MEA solvent system treating flue gases with a flow rate of about 47.000 Sm3/hr and a CO2 content of about 3.5%. For the base-case considered, the CO2 capture was about 90% and the thermal steam consumption was about 4.1 GJ/t-CO2. Emissions of MEA were very low and MEA related degradation products were all below detection levels, and all within the emission limits set by the Norwegian environmental authorities. The current work may be considered an independently verified baseline for a non-proprietary PCC amine-based solvent system.
The following process aspects were not considered in the current IVP work:
- Long-term performance indices such as heat exchanger fouling, mass transfer packing fouling, foaming, material corrosion, solvent quality control measures, solvent loss/replacement, etc.
- Use of anti-foam solution, which has proven to reduce the thermal steam consumptions at TCM DA
- Use of the installed lean vapor compressor system at TCM DA.
These aspects warrant further (IVC) work and studies in order to gain better understanding of the performance potential of the aqueous MEA solvent system as a non-proprietary PCC system.
Acknowledgements
The authors gratefully acknowledge the staff of TCM DA, Gassnova, Statoil, Shell, Sasol, and Aker Solutions for their contribution and work at the TCM DA facility.
The authors also gratefully acknowledge Gassnova, Statoil, Shell, and Sasol as the owners of TCM DA and Aker Solutions for their financial support and contributions.
Espen S. Hamborg et al. / Energy Procedia 63 (2014) 5994 – 6011 6009
Appendix A. Amine plant process information
Table 8 provides the amine plant main process information averaged over the base-case test time period. Process fluctuations, generally attributed to fluctuations in the CO2 content of the CHP flue gas, cannot be derived from the given values.
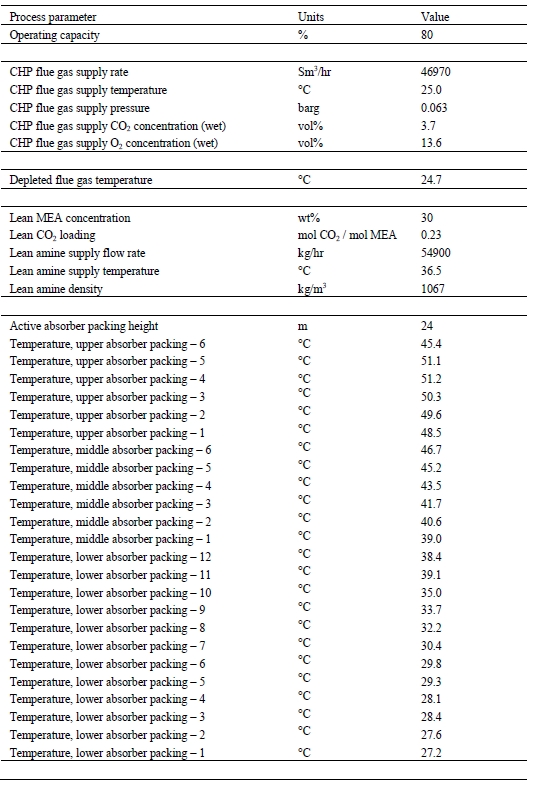
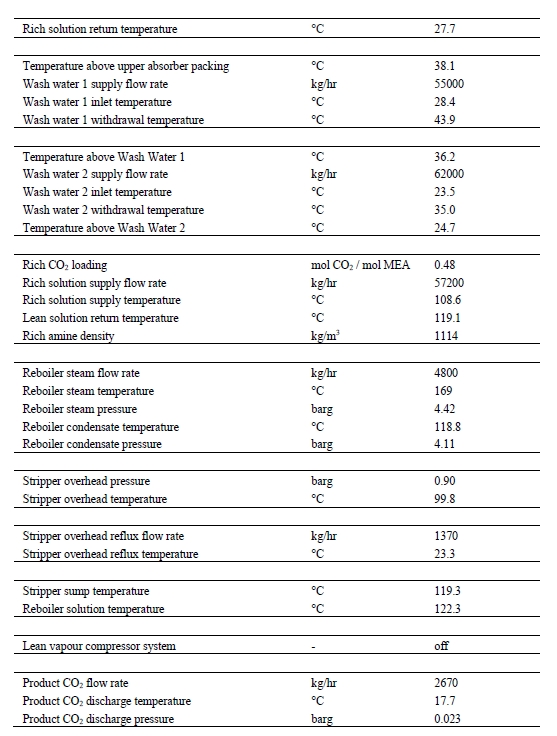
Table 8. Typical amine plant process information during Base-Case testing.
References
- Thimsen D, Maxson A, Smith V, Cents T, Falk-Pedersen O, Gorset O, Hamborg E S. Results from MEA testing at the CO2 Technology Centre Mongstad. Part I: Post-Combustion CO2 capture testing methodology. Energy Procedia; 2014.
- Brigman N, Shah M I, Falk-Pedersen O, Cents T, Smith V, De Cazenove T, Morken A K, Hvidsten O A, Chhaganlal M, Feste J K, Lombardo G, Bade O M, Knudsen J, Subramoney S C, Fostås B F, De Koeijer G, Hamborg E S. Results of amine plant operations from 30 wt% and 40 wt% aqueous MEA testing at the CO2 Technology Centre Mongstad. Energy Procedia; 2014.
- Morken A K, Nenseter B, Pedersen S, Chhaganlal M, Feste J K, Tyborgnes R B, Ullestad Ø, Ulvatn H, Zhu L, Mikoviny T, Wisthaler A, Cents T, Bade O M, Knudsen J, De Koeijer G, Falk-Pedersen O, Hamborg E S. Emission results of amine plant operations from MEA testing at the CO2 Technology Centre Mongstad. Energy Procedia; 2014.
- Knudsen J N, Andersen J, Jensen J N, Biede O. Evaluation of process upgrades and novel solvents for the post combustion CO2 capture process in pilot-scale. Energy Procedia 2011;4:1558-1565.

 End the search
End the search